Components & MOA

Bictegravir
- Novel and unboosted2
- Long plasma half-life of 17.3 hours1,†
-
Long integrase binding half-life of 38 hours in vitro3,‡
— The clinical relevance of these data has not been established
- Backed by over 2.8 million patient-years of experience§
+
A DHHS-recommended backbone
DESCOVY (FTC/TAF*) is a dual-NRTI that can be used as a backbone in 3 DHHS-recommended initial regimens for most people with HIV
DESCOVY is backed by over 4.1 million patient-years of experience4,||
BIKTARVY is DHHS recommended as an initial regimen for most people with HIV-15
TAF Provides Targeted Delivery of Tenofovir to HIV-Infected Cells1,6-8
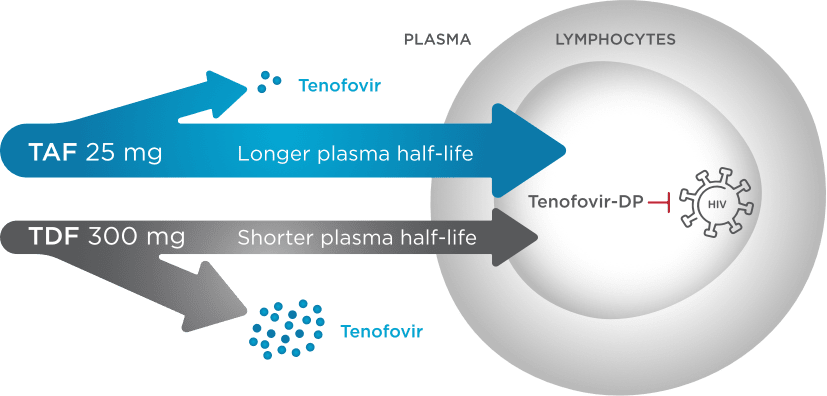
BIKTARVY contains TAF,¶ a novel prodrug of tenofovir (TFV) that efficiently delivers tenofovir into HIV-1 infected cells, including lymphocytes1,6
- TFV in the plasma is eliminated from the body through renal excretion6
- BIKTARVY is not recommended in patients with severe renal impairment (estimated CrCl <30 mL/min) except in virologically suppressed patients with CrCl <15 mL/min on chronic hemodialysis. BIKTARVY is not recommended for patients weighing ≥14 kg to <25 kg with CrCl <30 mL/min1
Less tenofovir in plasma
Tenofovir levels in plasma were up to 90% lower with TAF vs TDF6
A Dual-NRTI Backbone Is an Important Part of a Complete Regimen1,5,9-14
Triple therapy that combines a dual-NRTI backbone with an INSTI inhibits viral replication and can reduce the risk of ARV resistance5,9,10
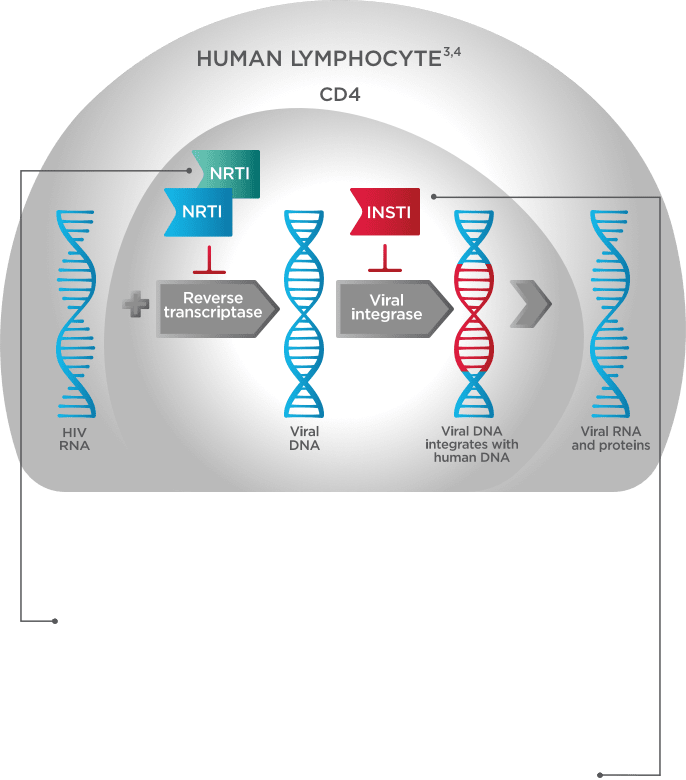
The two components of a dual-NRTI backbone# have been shown in vitro to work synergistically to block reverse transcriptase5,12-14
The third agent, an INSTI, inhibits the integration of viral DNA with human DNA1
#DHHS guidelines recommended dual-NRTI backbones include: FTC/TAF, FTC/TDF, and ABC/3TC.
3TC, lamivudine; ABC, abacavir; ARV, antiretroviral; CrCl, creatinine clearance; DP, diphosphate; DHHS, US Department of Health and Human Services; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; MOA, mechanism of action; NPA, National Prescription Audit; NRTI, nucleoside reverse transcriptase inhibitor; STR, single-tablet regimen; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
References: 1. BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2024. 2. Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12):7086-7097. 3. White K, Niedziela-Majka A, Novikov N, et al. Bictegravir dissociation half-life from HIV-1 G140S+Q148H integrase-DNA complexes. Poster presented at: Conference on Retroviruses and Opportunistic Infections; February 13-16, 2017; Seattle, WA. Poster 497. 4. Data on file. Gilead Sciences, Inc. 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Updated September 12, 2024. Accessed September 24, 2024. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf 6. Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1 positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449-455. 7. Margot NA, Ram RR, Abram ME, Haubrich R, Callebaut C. Antiviral activity of tenofovir alafenamide against HIV-1 with thymidine analog mutation(s) and M184V. Poster presented at: Conference on Retroviruses and Opportunistic Infections; March 4-7, 2018; Boston, MA. Poster 560. 8. Lee WA, He G-X, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898-1906. 9. National Institutes of Health. The HIV life cycle. Updated August 4, 2021. Accessed June 27, 2024. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-life-cycle 10. National Institutes of Health. FDA-approved HIV medicines. Updated March 23, 2023. Accessed June 27, 2024. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-aproved-hiv-medicines. 11. Laskey SB, Siliciano RF. A mechanistic theory to explain the efficacy of antiretroviral therapy. Nat Rev Microbiol. 2014;12(11):772-780. 12. Callebaut C, Stepan G, Tian Y, Miller MD. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob Agents Chemother. 2015;59(10):5909-5916. 13. Feng JY, Ly JK, Myrick F, et al. The triple combination of tenofovir, emtricitabine and efavirenz shows synergistic anti-HIV-1 activity in vitro: a mechanism of action study. Retrovirology. 2009;6:44. 14. Ray AS, Myrick F, Vela JE, et al. Lack of a metabolic and antiviral drug interaction between tenofovir, abacavir and lamivudine. Antivir Ther. 2005;10(3):451-457.


