47 (21-71)
Virologically Suppressed Patients
BIKTARVY®—Backed by Robust Clinical Trial Experience1
BIKTARVY was extensively studied across a diverse range of virologically suppressed patient populations1-3
On this page
- Study 1844: Virologically Suppressed Adults
- Study 1878: Virologically Suppressed Adults
- Study 4030: Virologically Suppressed Adults,
Including Those With Known or Suspected
Preexisting M184V/I Resistance Mutation - Study 1961: Virologically Suppressed Adult Women
- Study 4449: Virologically Suppressed Adults
Aged ≥65 - Study 1474: Virologically Suppressed Children and Adolescents
- BRAAVE: Virologically Suppressed Black American Adults
Additional analysis on this page:
Post Hoc Pooled Analysis: Virologically Suppressed People With M184V/I Resistance Mutation
Study 1844: Study Design
BIKTARVY vs ABC/DTG/3TC in virologically suppressed adults1,4,5
Phase 3, Randomized, Double-Blind, Active-Controlled Study
Study Conducted: November 2015—October 20196
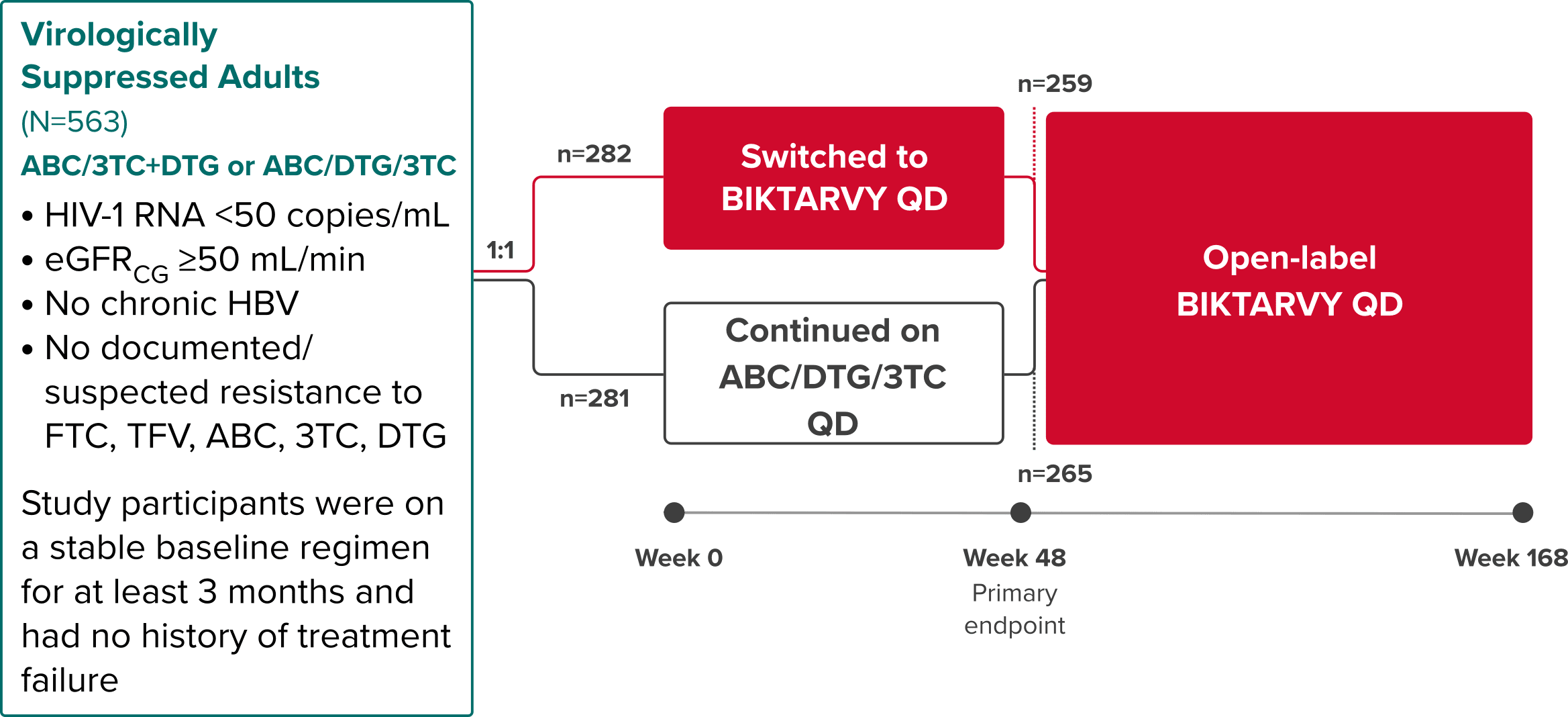
Primary endpoint
Proportion of adults with HIV-1 RNA ≥50 copies/mL at Week 48 (NI margin 4%) using the FDA snapshot algorithm4
Open-label extension
The objective of the OLE from Week 48 through Week 168 was to evaluate the efficacy and safety of BIKTARVY after additional exposure5
- Efficacy in the extension phase from Week 48 through Week 168 was calculated using a missing=excluded (M=E) analysis
Study 18441
BIKTARVY (n=282)
ABC/DTG/ 3TC (n=281)
Median age, years (range)
45 (20-70)
88
90
12
10
73
73
21
22
3
3
Hispanic/Latino, %
16
19
Median eGFRCG, mL/min (IQR)
101 (85-119)
101 (85-122)
Retrospective Analysis of Preexisting Resistance at Baseline in Studies 1844 & 18782,*,†
BIKTARVY Arms From Studies 1844 & 1878 N‡=519 (IN) N‡=543 (RT/PR)
INSTI, %
2.5
NRTI, %
16.4
NNRTI, %
22.8
PI, %
10.1
1.7
TAMs, %
6.4
11.8
0.2
9.9
4.6
1.3
- Historical genotype records were assessed at screening, and documented resistance to study drugs or evidence of previous virologic failure led to exclusion. Retrospective deep sequencing analysis was later attempted on baseline samples from all BIKTARVY-treated participants.
*This list is not inclusive of all RAMs observed in this analysis.2
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.3
‡Retrospective deep sequencing data were combined with the population sequencing data from screening.
3TC, lamivudine; ABC, abacavir; CD4, cluster of differentiation 4; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); IN, integrase; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PR, protease; RAM, resistance-associated mutation; RT, reverse transcriptase; TAM, thymidine-associated mutation.
References:
- Molina J-M, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, Phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357-e365.
- Andreatta K, Willkom M, Martin R, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J Antimicrob Chemother. 2019;74(12):3555-3564.
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
Study 1844: Efficacy
Durable efficacy in virologically suppressed adults at 3 years1,4,5
94% of adults who switched to BIKTARVY maintained virologic suppression at Week 48
Virologic Response in Study 18441,4,*

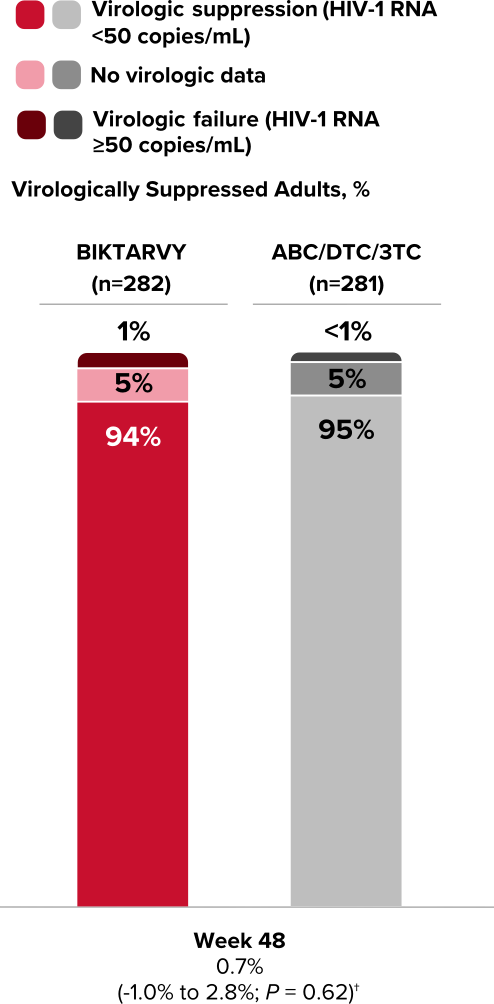
*Percentages do not total 100% due to rounding.
†Treatment difference in HIV-1 RNA ≥50 copies/mL (95% confidence interval, P value).
Durable viral suppression at Week 1685
In a 120-week open-label extension from Week 48, using an M=E analysis, virologic suppression was maintained in 100% of study participants on BIKTARVY (n=14) at Week 168 from BIKTARVY switch.5
- In an M=E analysis, study participants with missing data are excluded when calculating the proportion of participants with HIV-1 RNA <50 copies/mL
IMPORTANT SAFETY INFORMATION (cont’d)
Contraindications
- Coadministration: Do not use BIKTARVY with dofetilide or rifampin.
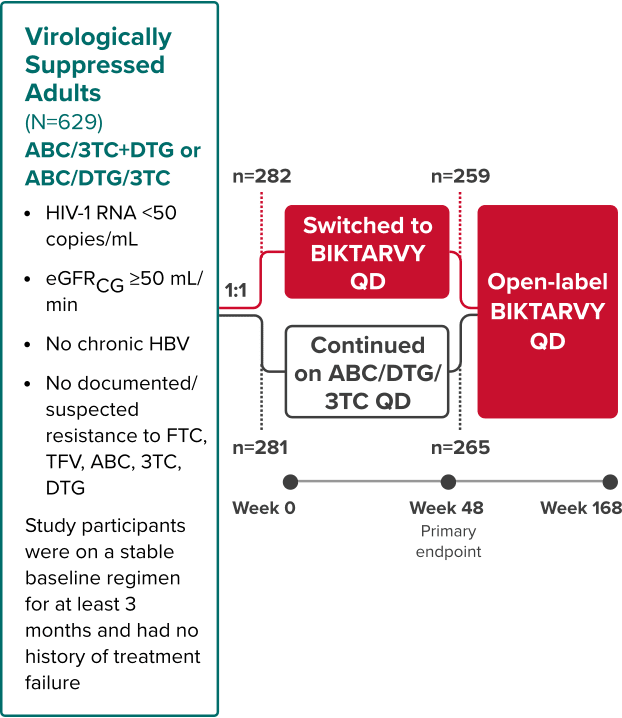
Study 1878: Study Design
BIKTARVY vs ATV- or DRV-based regimen in virologically suppressed adults1,7,8
Phase 3, Randomized, Open-Label, Active-Controlled Study
Study Conducted: November 2015—December 20199


‡Cobicistat or ritonavir.
Primary endpoint
Proportion of adults with HIV-1 RNA ≥50 copies/mL at Week 48 (NI margin 4%) using the FDA snapshot algorithm7
Extension phase
The objective of the extension phase from Week 48 through Week 96 was to evaluate the efficacy and safety of BIKTARVY after additional exposure8
- Efficacy in the extension phase from Week 48 through Week 96 was calculated using a missing=excluded (M=E) analysis
Study 18781
BIKTARVY (n=290)
ATV- or DRV- based
Regimen
(n=287)
Median age, years (range)
48 (20-74)
47 (21-79)
84
82
16
18
65
66
27
25
2
3
Hispanic/Latino, %
21
16
Median eGFRCG, mL/min (IQR)
107 (87.0-124.2)
105 (87.1-125.3)
ABC/3TC, %
84, 16
85, 15
57, 43
54, 46
Retrospective Analysis of Preexisting Resistance at Baseline in Studies 1844 & 18782,*,†
BIKTARVY Arms From Studies 1844 & 1878 N‡=519 (IN) N‡=543 (RT/PR)
INSTI, %
2.5
NRTI, %
16.4
NNRTI, %
22.8
PI, %
10.1
1.7
TAMs, %
6.4
11.8
0.2
9.9
4.6
1.3
- Historical genotype records were assessed at screening, and documented resistance to study drugs or evidence of previous virologic failure led to exclusion. Retrospective deep sequencing analysis was later attempted on baseline samples from all BIKTARVY-treated participants.
*This list is not inclusive of all RAMs observed in this analysis.2
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.3
‡Retrospective deep sequencing data were combined with the population sequencing data from screening.
3TC, lamivudine; ABC, abacavir; ATV, atazanavir; CD4, cluster of differentiation 4; DRV, darunvavir; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); FTC, emtricitabine; IN, integrase; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PR, protease; RAM, resistance-associated mutation; RT, reverse transcriptase; TAF, tenofovir alafenamide; TAM, thymidine-associated mutation; TDF, tenofovir disoproxil fumarate.
References:
- Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347-e356.
- Andreatta K, Willkom M, Martin R, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J Antimicrob Chemother. 2019;74(12):3555-3564.
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
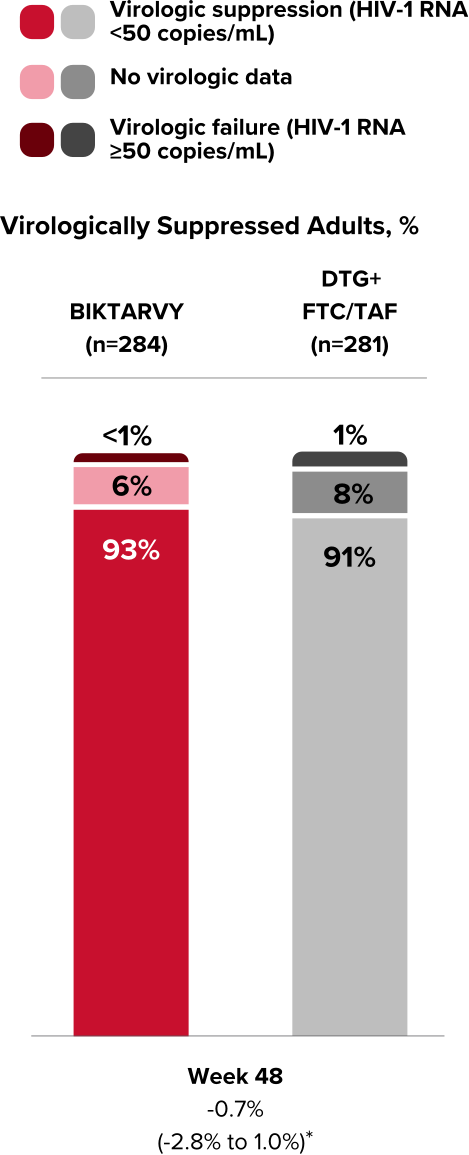
Study 1878: Efficacy
Powerful efficacy in virologically suppressed adults at 2 years1,7,8
92% of adults who switched to BIKTARVY maintained virologic suppression at Week 48
Virologic Response in Study 18781,7

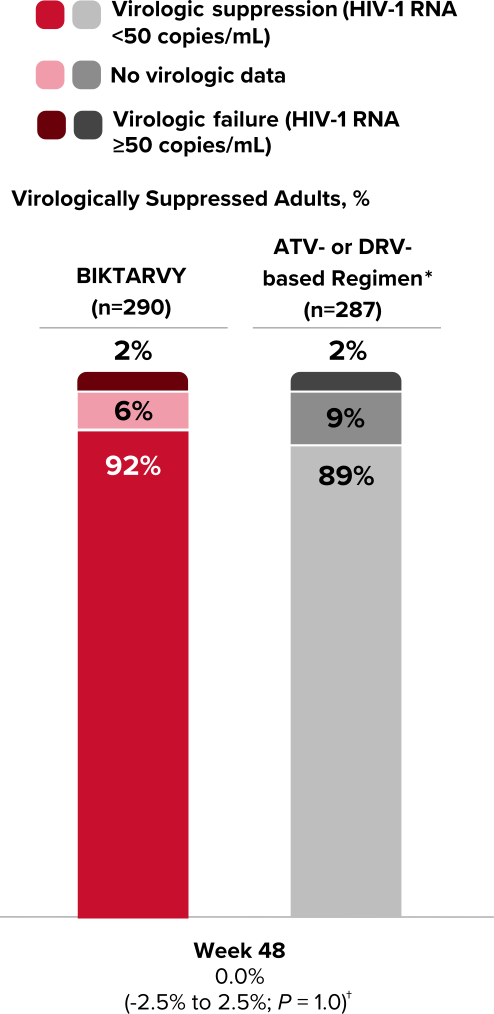
*ABC/3TC or FTC/TDF + boosted ATV or DRV (cobicistat or ritonavir) regimen.
†Treatment difference in HIV-1 RNA ≥50 copies/mL (95% confidence interval, P value).
Durable viral suppression at Week 968
In the extension from Week 48, using an M=E analysis, virologic suppression was maintained in 100% of study participants on BIKTARVY (n=318) at Week 96 from BIKTARVY switch.8
- In an M=E analysis, participants with missing data are excluded when calculating the proportion of participants with HIV-1 RNA <50 copies/mL
Studies 1844 and 1878: Resistance
Zero cases of resistance to BIKTARVY through 3 years in virologically suppressed adults1,4,5,7,8,10,‡
Among 572 virologically suppressed adults randomized to BIKTARVY in Studies 1844 and 1878 through Week 48, 2 participants with virologic rebound had genotypic and phenotypic data (1 for RT, 1 for IN and RT). No treatment-emergent resistance to BIKTARVY was detected through Week 48.1,4,7,10
In the extension phases:
- Study 1844 open-label extension: 524 virologically suppressed adults participated; 259 were continuing BIKTARVY and 265 switched to BIKTARVY at Week 48. No treatment-emergent resistance to BIKTARVY was detected through Week 168, including in the 1 participant who switched to BIKTARVY and met the criteria for resistance analysis.5,11
- Study 1878 extension: 516 virologically suppressed adults participated; 272 were continuing BIKTARVY and 244 switched to BIKTARVY at Week 48. No treatment-emergent resistance to BIKTARVY was detected through Week 96, including in the 3 participants who switched to BIKTARVY and met the criteria for resistance analysis.8,12
‡Based on the resistance analysis population.
IMPORTANT SAFETY INFORMATION (cont’d)
Warnings and precautions
- Drug interactions: See Contraindications and Drug Interactions sections. Consider the potential for drug interactions prior to and during BIKTARVY therapy and monitor for adverse reactions.
- Immune reconstitution syndrome, including the occurrence of autoimmune disorders with variable time to onset, has been reported.
Study 4030: Study Design
BIKTARVY vs DTG+FTC/TAF in virologically suppressed adults with HIV, including those with NRTI resistance1,13
Phase 3, Randomized, Double-Blind, Active-Controlled Study
Study Conducted: June 2017−February 202114


Primary endpoint
Proportion of adults with HIV-1 RNA ≥50 copies/mL at Week 48 (NI margin 4%) using FDA snapshot algorithm13
Secondary endpoint
Proportion of adults with HIV-1 RNA <50 copies/mL at Week 48 (NI margin 10%) using FDA snapshot algorithm13
Study design randomization
Randomization was stratified by NRTI backbone at screening (FTC/TAF vs FTC/TDF) and documented or suspected history of NRTI resistance.
Study 40301
BIKTARVY (n=284)
DTG+FTC/
TAF
(n=281)
Median age, years (range)
51 (22-79)
50 (20-79)
86
85
14
15
71
72
24
22
3
5
1
1
Pacific Islander, %
1
<1
Hispanic/Latino, %
22
18
Median eGFRCG, mL/min (IQR)
97 (79-114)
100 (83-124)
HIV/HBV coinfection, %
5
HIV/HCV coinfection, %
<1
84, 16
85, 15
57, 43
54, 46
BMI, kg/m2, median (IQR)
26 (24-31)
27 (24-31)
References:
- Sax PE, Rockstroh JK, Luetkemeyer AF, et al; GS-US-380-4030 Investigators. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis. 2021;73(2):e485-e493.
Frequency of Baseline Primary Resistance-Associated Substitutions in Study 4030 1, * ,†
n (%) ‡
BIKTARVY (n=284)
DTG+FTC/
TAF (n=281)
NRTI
63 (26%)
55 (24%)
47 (20%)
34 (15%)
3 (1%)
2 (1%)
16 (7%)
16 (7%)
16 (7%)
17 (7%)
NNRTI
61 (26%)
57 (25%)
37 (16%)
28 (12%)
5 (2%)
12 (5%)
PI
15 (6%)
23 (10%)
INSTI
15 (7%)
5 (3%)
8 (4%)
4 (2%)
2 (1%)
0 (0%)
1 (0.5%)
0 (0%)
*This list is not inclusive of all RAMs observed in the analysis.1
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.2
‡The denominators for analysis are the number of participants with available data for PR/RT (238 for BIKTARVY arm and 232 for the DTG+FTC/TAF arm) and IN (213 for the BIKTARVY arm and 200 for the DTG+FTC/TAF arm).
Historical and retrospective proviral baseline data, if available, were combined for baseline resistance analyses1
- At screening, 50% (n=285/565) of participants had historical genotypes of plasma HIV-1 RNA available1
- 69% (n=391/565) of participants had results available from retrospective baseline proviral DNA genotypes from participants with whole blood samples (n=478) using next generation sequencing of the HIV-1 polymerase region1,§
- Overall, 83% (n=470/565) of participants had baseline genotypic data available1
§Many assay failures were because of insufficient sample volume.
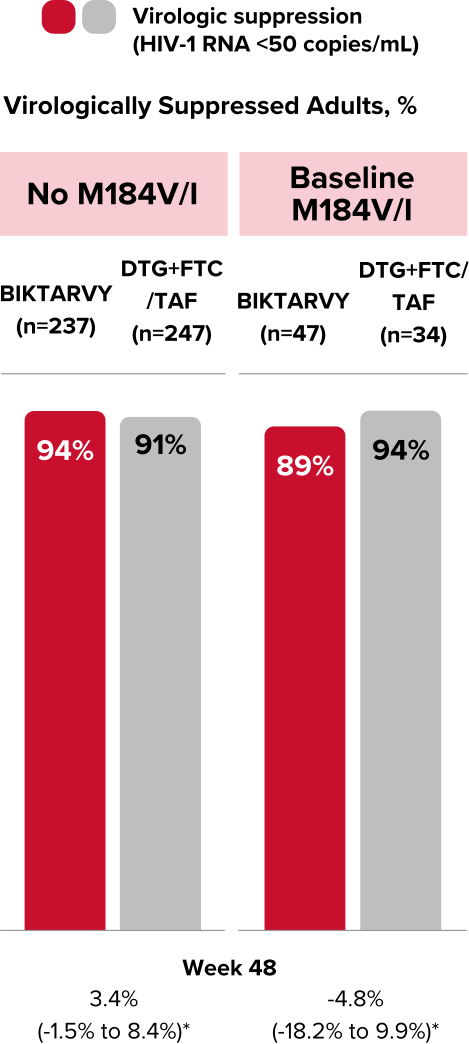
Preexisting M184V/I Resistance Mutation at Baseline
Frequency of Baseline M184V/I Resistance Mutation in Participants With RT/PR data1,||
Overall (n=470)
BIKTARVY (n=238)
DTG+FTC/TAF (n=232)
M184V/I,
n (%)
81 (17%)
47 (20%)
34 (15%)
41 (9%)
22 (9%)
19 (8%)
57 (12%)
35 (35%)
22 (9%)
||Substitutions were detected by historical genotype, retrospective proviral DNA archive genotype, or both.
References:
- Acosta RK, Willkom M, Andreatta K, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) from dolutegravir (DTG)+F/TAF or DTG+F/tenofovir disoproxil fumarate (TDF) in the presence of pre-existing NRTI resistance. J Acquir Immune Defic Syndr. 2020;85(3):363-371.
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
Predictors of Preexisting M184V/I Resistance Mutation by Post Hoc Analysis
Statistically Significant Independent Predictors of Preexisting M184V/I Resistance1,†
M184V/I Present
Odds Ratio
(95% CI)1.1
(1.1-1.2)<0.0001
2.2
(1.1-4.3)0.0189
2.5
(1.4-4.6)0.0026
2.6
(1.1-6.0)0.0295
2.7
(1.5-4.7)0.0007
*Stepwise logistic regression was conducted (n=470 with a historical or proviral genotype). The significance level for entry into the model = 0.20: the significance level for staying in the model = 0.05.
†The predictors considered for model selection were intrinsic factors (age group, sex, race group, ethnicity group, BMI category, CKD stage, and region [US vs non-US]) and HIV-specific factors at baseline (CD4 count category, HIV-1 RNA category, HIV acquisition risk factor, HIV disease status, time since ART start, prior use of each class of ART third agent, number of prior agents, baseline ARV regimen and its duration, and PI or NNRTI resistance at baseline).
References:
- Acosta RK, Willkom M, Andreatta K, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) from dolutegravir (DTG)+F/TAF or DTG+F/tenofovir disoproxil fumarate (TDF) in the presence of pre-existing NRTI resistance. J Acquir Immune Defic Syndr. 2020;85(3):363-371.
Study 4030: Resistance
Zero cases of resistance to BIKTARVY through 48 weeks, including those with preexisting M184V/I resistance mutation13,‡
Among the 284 virologically suppressed adults randomized to BIKTARVY in Study 4030 through Week 48, 0 participants met the criteria for resistance analysis, and no treatment-emergent resistance to BIKTARVY was detected through Week 48.13
‡Based on the final resistance analysis population.
IMPORTANT SAFETY INFORMATION (cont’d)
Warnings and precautions
- Drug interactions: See Contraindications and Drug Interactions sections. Consider the potential for drug interactions prior to and during BIKTARVY therapy and monitor for adverse reactions.
- Immune reconstitution syndrome, including the occurrence of autoimmune disorders with variable time to onset, has been reported.
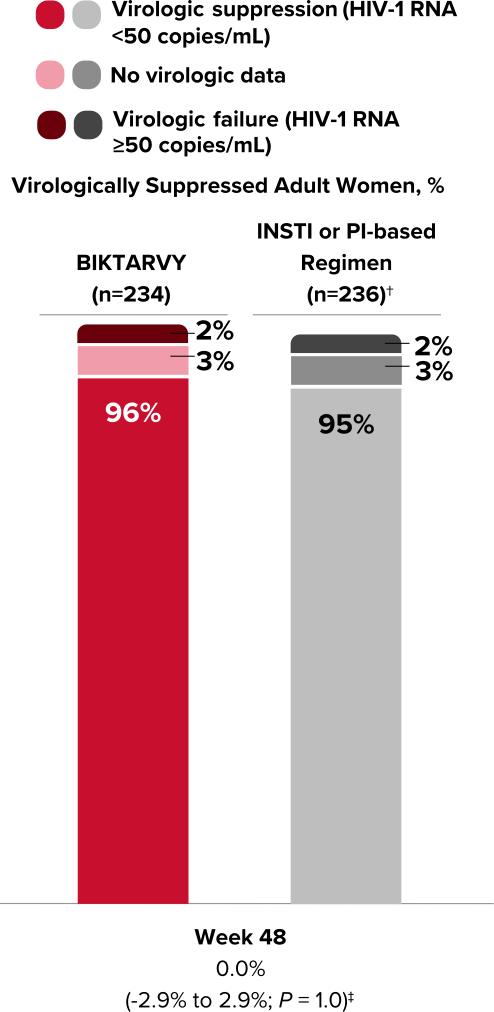
Study 1961: Study Design
BIKTARVY vs an INSTI- or PI-based regimen in virologically suppressed adult women2,15
Phase 3, Randomized, Open-Label, Active-Controlled Study
Study Conducted: February 2016—November 201816

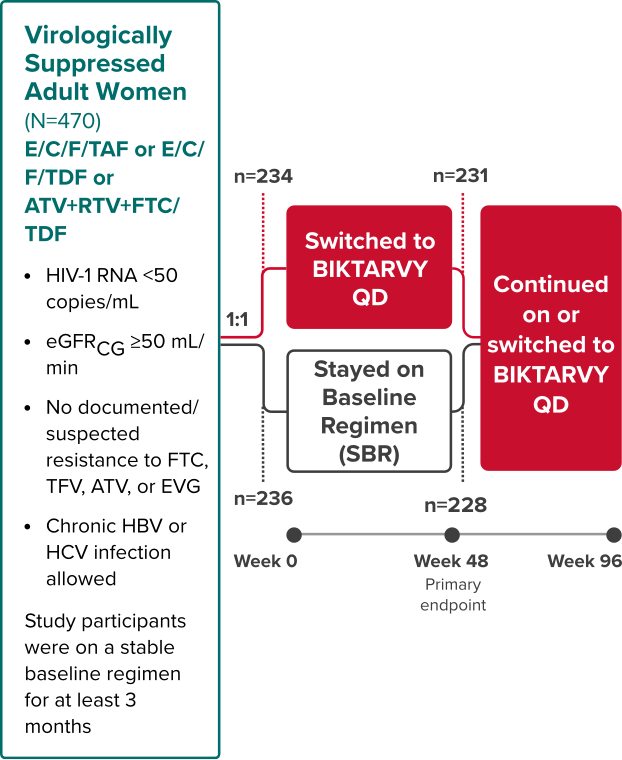
Primary endpoint
Proportion of participants with HIV-1 RNA ≥50 copies/mL at Week 48 (NI margin 4%) using the FDA snapshot algorithm15
Extension phase
The objective of the extension phase from Week 48 through Week 96 was to evaluate the efficacy and safety of BIKTARVY after additional exposure2
- At Week 48, the women receiving an INSTI- or PI-based regimen at baseline were switched to BIKTARVY, and an additional analysis was performed at Week 96
- Efficacy in the extension phase from Week 48 through Week 96 was calculated using a missing=excluded (M=E) analysis
Study 19611,2: Selected Population Characteristics
BIKTARVY (n=234)
INSTI- or PI-
based Regimen
(n=236)
Median age, years (range)
39 (21-63)
40 (20-63)
100
100
39
35
28
28
21
23
12
14
Hispanic/
Latino, %
15
16
Median eGFRCG, mL/min (IQR)
99.6 (82.8-115.8)
102.0 (85.8-124.5)
53
53
42
42
FTC/TDF, %
5
6
ARV, antiretroviral; ATV, atazanavir; C, cobicistat; E, elvitegravir; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); F, emtricitabine; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; IQR, interquartile range.
References:
- Kityo C, Hagins D, Koenig E, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, Phase 3, noninferiority trial. J Acquir Immune Defic Syndr. 2019;82(3):321-328.
- Data on file. Gilead Sciences, Inc.
Study 1961: Resistance
Zero cases of resistance to BIKTARVY through 2 years in virologically suppressed adult women2,15,§
Among 234 virologically suppressed adult women randomized to BIKTARVY in Study 1961 through Week 4815:
- 1 participant met the criteria for resistance analysis
- No treatment-emergent resistance to BIKTARVY was detected through Week 48
459 virologically suppressed adult women participated in the extension phase of Study 1961. 231 of them continued on BIKTARVY and 228 switched to BIKTARVY at Week 482:
- No treatment-emergent resistance to BIKTARVY was detected through Week 96, including in the 3 participants who switched to BIKTARVY and met the criteria for resistance analysis
§Based on the final resistance analysis population.
IMPORTANT SAFETY INFORMATION (cont’d)
Adverse reactions
- Most common adverse reactions (incidence ≥5%; all grades) in clinical studies through week 144 were diarrhea (6%), nausea (6%), and headache (5%).
Study 4449: Study Design
BIKTARVY in virologically suppressed adults aged ≥65 years1,17,18
Phase 3b, Open-Label, Single-Arm Study
Study Conducted: March 2018—May 202019
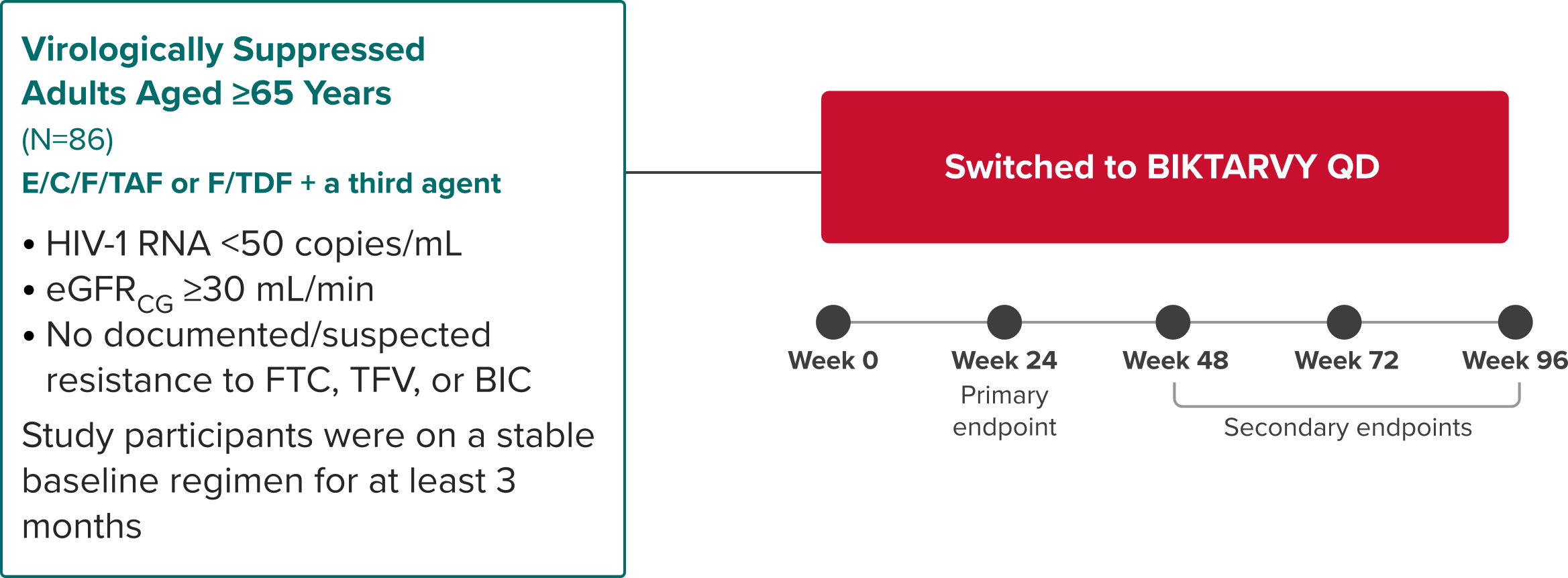
Primary endpoint
Proportion of participants with HIV-1 RNA <50 copies/mL at Week 24 using the FDA snapshot algorithm17
Secondary endpoints
- Efficacy at Weeks 48, 72, and 96 using the FDA snapshot algorithm18
- Safety and tolerability through Week 9618
Study 44491: Selected Population Characteristics
BIKTARVY
(n=86)
Median age, years (range)
69 (65-80)
87
13
95
1
4
Hispanic/Latino, %
14
Median eGFRCG, mL/min (IQR)
76 (39.6-130.2)
92
5
1
1
1
Retrospective Analysis of Preexisting Resistance at Baseline1,2*,†
Participants Switching to BIKTARVY With IN (n=83) or RT/PR (n=84) Data
1 (1.2%)
9 (10.7%)
10 (11.9%)
1 (1.2%)
9 (10.7%)
4 (4.8%)
3 (3.6%)
3 (3.6%)
Preexisting resistance was evaluated by historical genotypes, if available, and retrospective proviral DNA genotype testing performed on baseline samples. Participants with resistance to BIC, FTC, or TFV were excluded.1
*This list is not inclusive of all RAMs observed in the analysis.
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.3
C, cobicistat; E, elvitegravir; EFV, efavirenz; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); F, emtricitabine; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
References:
- 1. Maggiolo F, Rizzardini G, Molina J-M, et al. Bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people with HIV aged ≥65 years: week 48 results of a Phase 3b, open-label trial. Infect Dis Ther. 2021;10(2):775-788.
- Maggiolo F, Rizzardini G, Molina J-M, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in adults aged 65 years or older: Week 96 results from an international, Phase 3b, open-label trial (GS-US-380-4449). Poster presented at: IAS 2021; July 18-21, 2021; Virtual. Poster PEB160.
- BIKTARVY Prescribing Information. Gilead Sciences, Inc.; 2025.
Study 4449: Resistance
Zero cases of resistance to BIKTARVY through 2 years in virologically suppressed adults aged ≥65 years17,18,||
Among 86 virologically suppressed adults aged ≥65 years in Study 4449 through Week 9617,18,§:
- No treatment-emergent resistance to BIKTARVY was detected through Week 96. No participants met the criteria for resistance analysis
||Based on the resistance analysis population.
IMPORTANT SAFETY INFORMATION (cont’d)
Drug interactions
- Prescribing information: Consult the full prescribing information for BIKTARVY for more information on Contraindications, Warnings, and potentially significant drug interactions, including clinical comments.
Study 1474: Study Design
BIKTARVY in virologically suppressed children and adolescents1,21,22
Phase 2/3, Single-Arm, Open-Label, Multi-Cohort Study
Study Conducted: September 2016—November 2026 (Estimated Completion)23
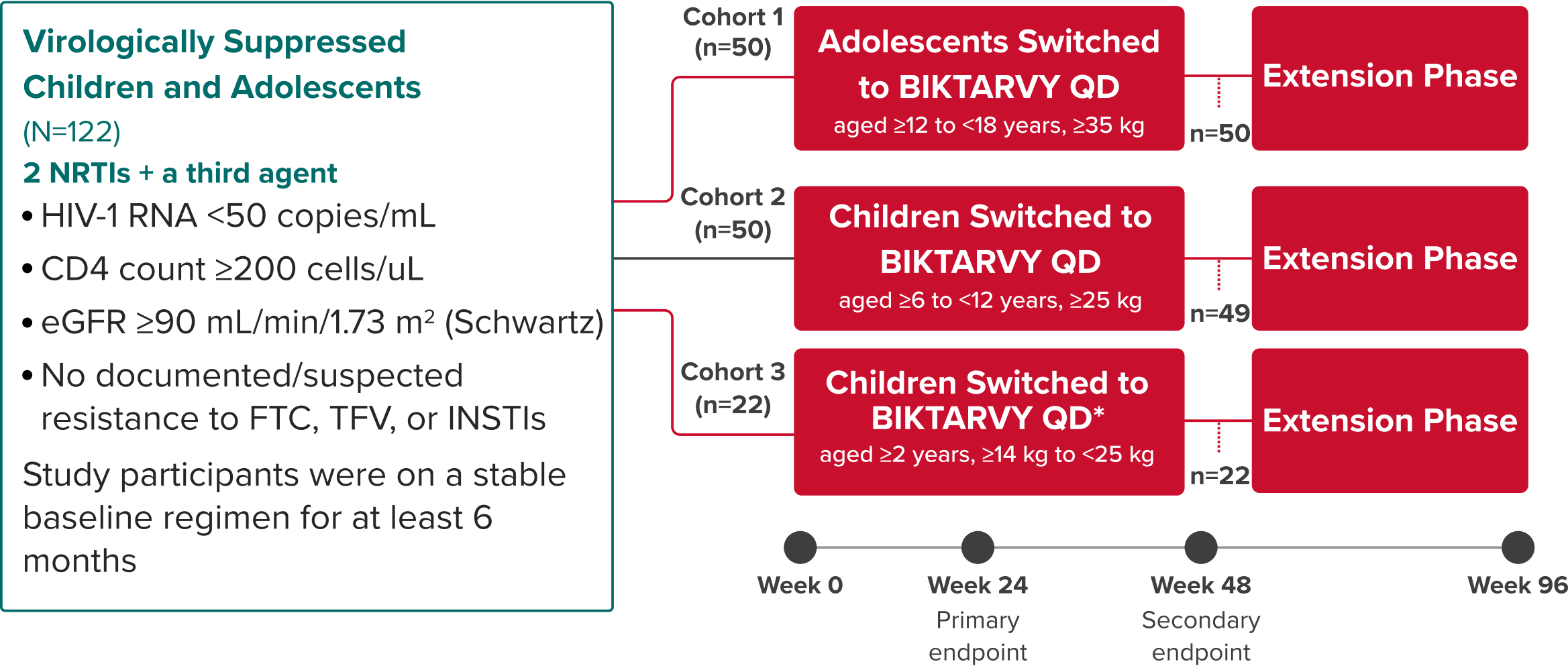
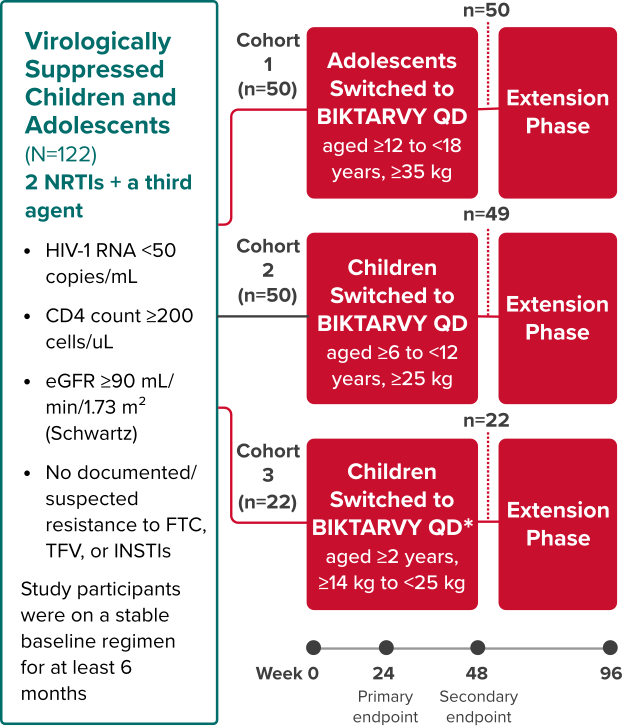
*Cohort 3 received low-dose BIKTARVY (bictegravir 30 mg, emtricitabine 120 mg, tenofovir alafenamide 15 mg).
Primary endpoint
Determination of plasma PK and evaluation of safety and tolerability through Week 241,21,22
Secondary endpoints
Proportion of children and adolescents with HIV-1 RNA <50 copies/mL at Week 24 using the FDA snapshot algorithm22
- Efficacy, safety, and tolerability were also evaluated at Week 48
Extension phase (cohorts 1 & 2)
Efficacy in the extension phase from Week 48 to Week 96 was calculated using missing=excluded (M=E) and missing=failure (M=F) analyses22
- Safety and tolerability were also evaluated through Week 96
Study 14741-3: Selected Population Characteristics
Cohort 1
(aged ≥12 to <18
years, ≥35 kg;
n=50)
Cohort 2
(aged ≥6 to <12
years, ≥25 kg;
n=50)
Cohort 3
(≥2 years, ≥14 kg
to <25 kg; n=22)
Median age, years (range)
15 (12-17)
10 (6-11)
6 (3-9)
36
46
50
64
54
50
65
72
73
27
22
23
Other Pacific Islander, %
2
0
0
2
4
0
4
2
5
Hispanic/
Latino, %
4
0
eGFR (Schwartz), mL/min (IQR)
145 (134.0-170.0)
153.5 (144.0-173.0)
160.5 (145.0-168.0)
70
92
48
72
Retrospective Analysis of Preexisting Resistance at Baseline4*,†
n (%)‡
Cohorts 1 & 2 (n=100)
Cohort 3 (n=22)
NRTI
16
(20%)1
(6.7%)11
(13.75%)1
(6.7%)10
(12.5%)1
(6.7%)1
(1.25%)0
NNRTI
26
(32.5%)2
(13.3%)6
(7.5%)0
9
(11.25%)2
(13.3%)PI
8
(10%)1
(6.7%)INSTI
4
(5.1%)2
(14.3%)0
2
(14.3%)1
(1.3%)0
Preexisting drug resistance was assessed using available historical genotypes or retrospective proviral DNA genotyping of baseline samples. Participants with any known or suspected resistance to FTC, TFV, or INSTIs were excluded at screening.2
*This list is not inclusive of all RAMs observed in this analysis.
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.1
‡The denominators for analysis are the number of participants with available data for PR/RT (80 for Cohorts 1 & 2 and 15 for Cohort 3) and IN (78 for Cohorts 1 & 2 and 14 for Cohort 3).4
FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RAM, resistance-associated mutation; TAF, tenofovir alafenamide; TAM, thymidine-associated mutation; TFV, tenofovir.
References:
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
- Gaur AH, Cotton MF, Rodriguez CA, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide in adolescents and children with HIV: week 48 results of a single-arm, open-label, multicentre, phase 2/3 trial. Lancet Child Adolesc Health. 2021;5(9):642-651.
- Natukunda E, Rodriguez CA, McGrath EJ, et al. B/F/TAF in virologically suppressed adolescents and children: two-year outcomes in 6 to <18 year olds and six-month outcomes in toddlers. Abstract presented at: International Workshop on HIV & Pediatrics. July 16-17, 2021; Virtual. Abstract 2.
- Data on file. Gilead Sciences, Inc.
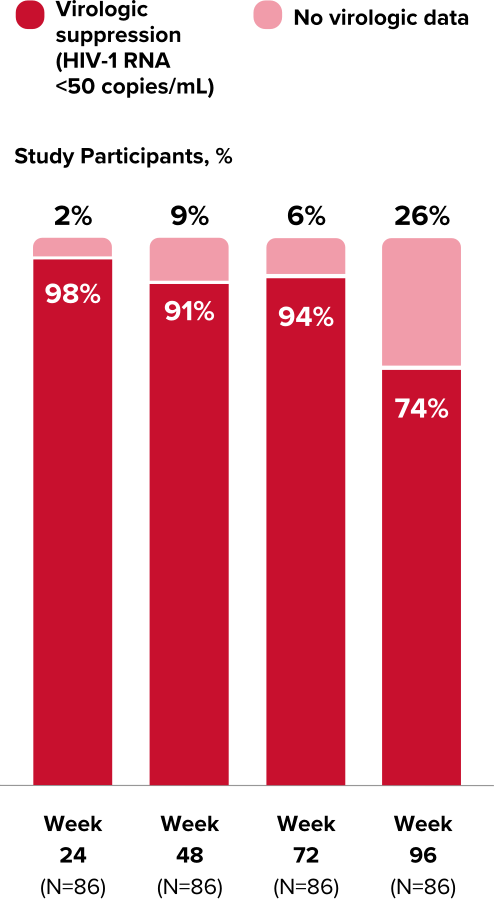
Study 1474: Efficacy
Powerful efficacy in virologically suppressed children and adolescents at 2 years1,21,22,24
Virologic Response in Study 1474

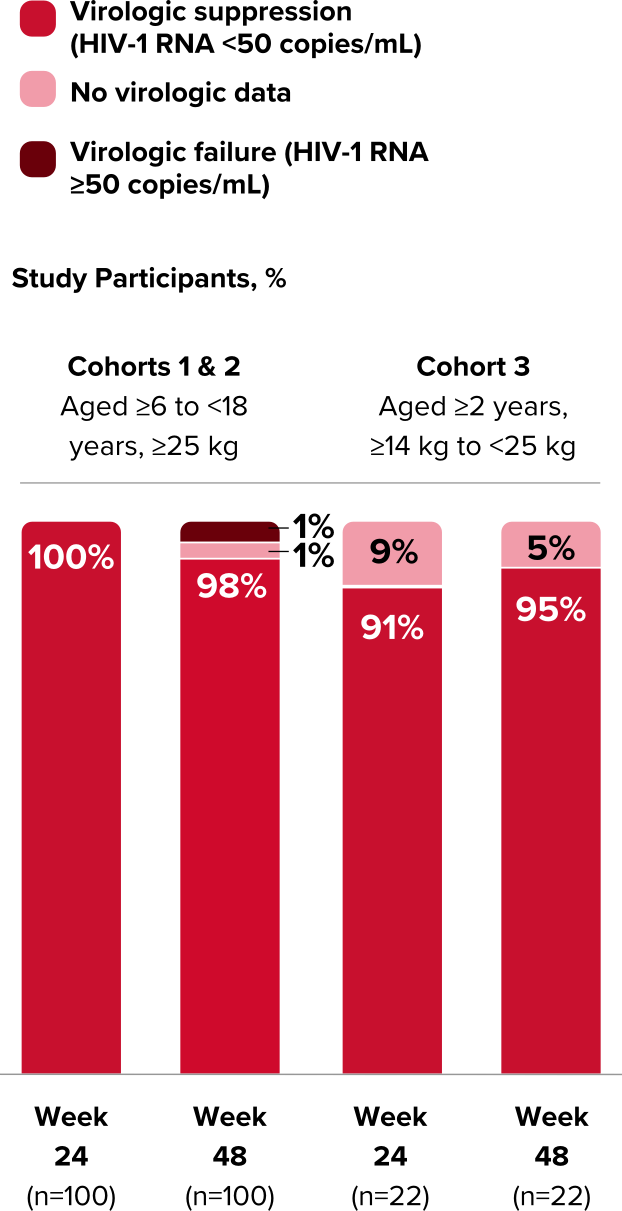
- High rates of virologic suppression were reported using the FDA snapshot algorithm
- Some plasma PK parameters were lower in cohort 1 and higher in cohorts 2 and 3 relative to adults, but these differences were not considered clinically significant1,*
*Mean BIC Ctrough was lower in cohort 1 and mean BIC Cmax and FTC and TAF exposures (AUCtau and Cmax) were higher in cohorts 2 and 3 relative to adults.
Durable viral suppression at Week 96 (cohorts 1 & 2)1,12,21,22
In the extension from Week 48, using an M=E analysis, 99% of children and adolescents (n=96) maintained virologic suppression at Week 96. Using an M=F analysis, 95% (n=100) maintained virologic suppression at Week 96.
- In an M=E analysis, study participants with missing data are excluded when calculating the proportion of participants with HIV-1 RNA <50 copies/mL. In an M=F analysis, all missing data are treated as treatment failures (HIV-1 RNA ≥50 copies/mL).
Study 1474: Resistance
Zero cases of resistance to BIKTARVY through Week 96 in virologically suppressed children and adolescents1,12,21,22,24,¶
Among 100 virologically suppressed children and adolescents aged >6 to <18 years in Study 1474 (cohorts 1 & 2)1,12,21,22:
- One participant met criteria for resistance analysis. No treatment-emergent resistance to BIKTARVY was detected through Week 96
Among 22 virologically suppressed children aged ≥2 (weighing ≥14 kg to <25 kg) in Study 1474 (cohort 3)1,22,24:
- No treatment-emergent resistance to BIKTARVY was detected through Week 48. No participants met the criteria for resistance analysis
¶Based on the resistance analysis population.
IMPORTANT SAFETY INFORMATION (cont’d)
Pregnancy and lactation
- Pregnancy: BIKTARVY is recommended in pregnant individuals who are virologically suppressed on a stable ARV regimen with no known substitutions associated with resistance to any of the individual components of BIKTARVY. Lower plasma exposures of BIKTARVY were observed during pregnancy; therefore, viral load should be monitored closely during pregnancy. An Antiretroviral Pregnancy Registry (APR) has been established. Available data from the APR for BIC, FTC, or TAF show no difference in the rates of birth defects compared with a US reference population.
- Lactation:Individuals infected with HIV-1 should be informed of the potential risks of breastfeeding.
BRAAVE: Study Design
BIKTARVY vs stable baseline regimen in virologically suppressed Black Americans3,25,26
Randomized Phase 3b, Multicenter, Open-Label Study
Study Conducted: August 2018—August 202027
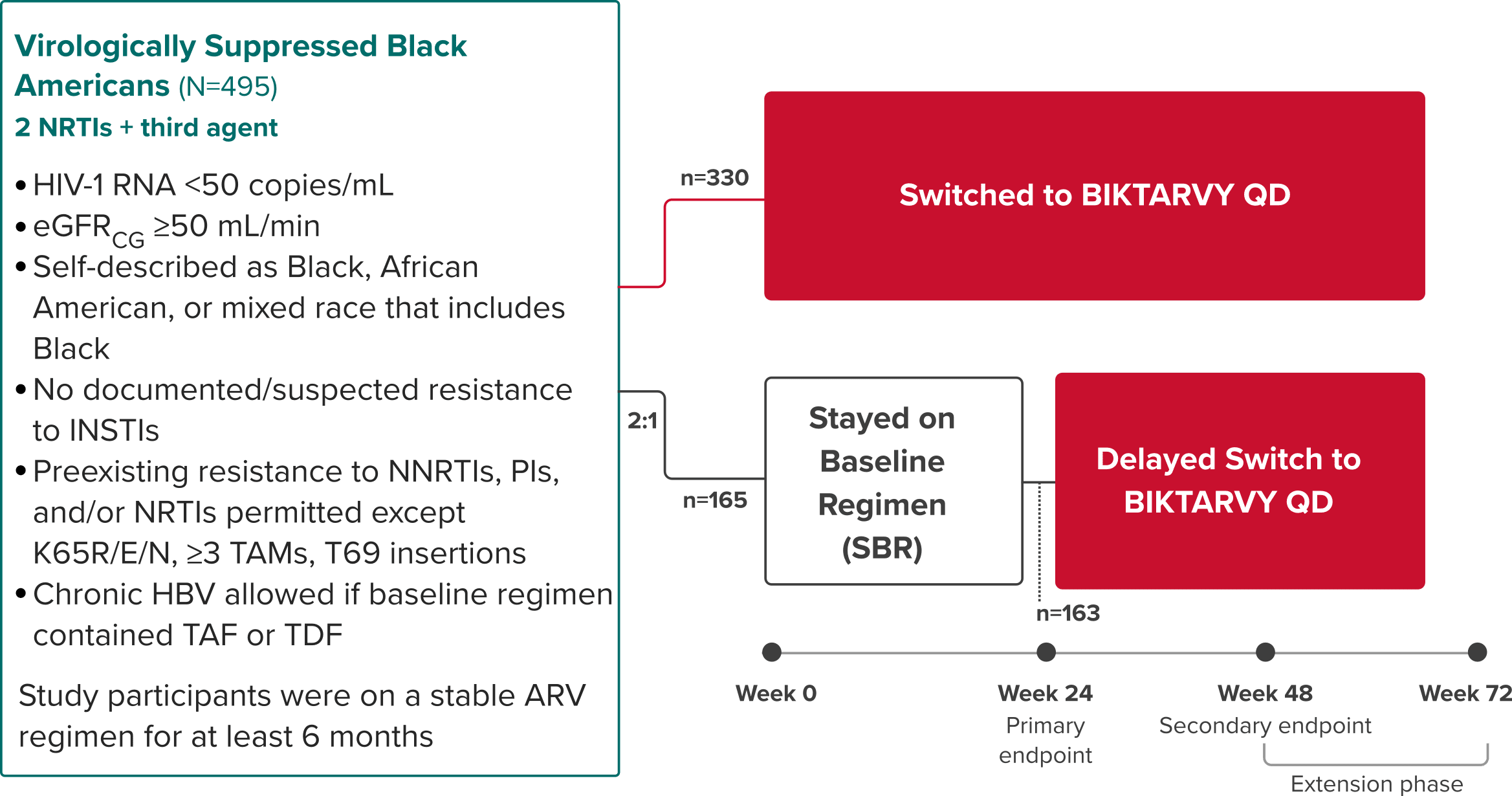
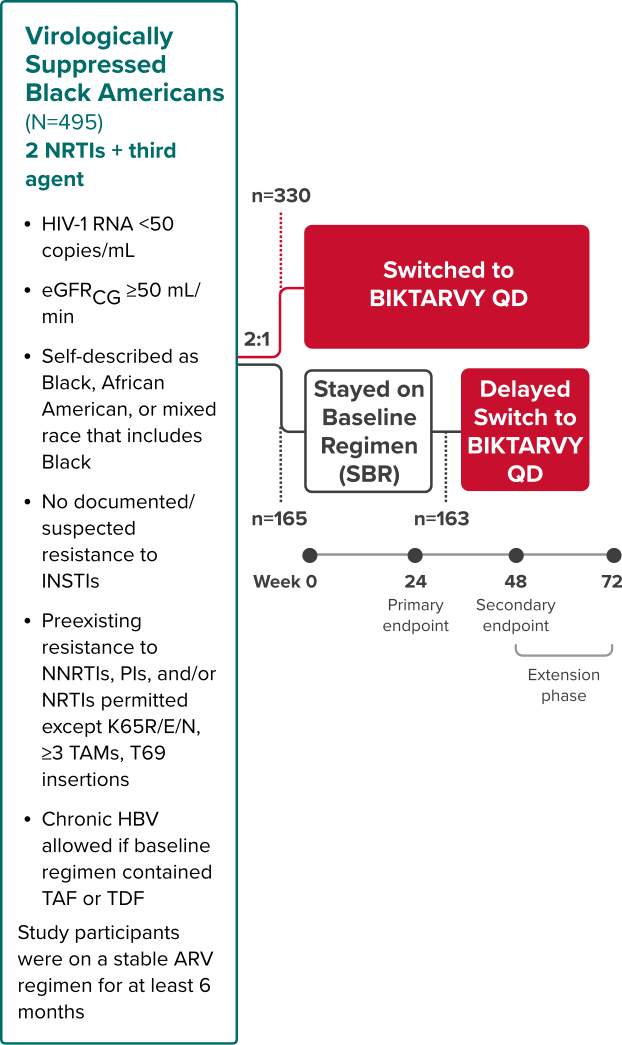
Primary endpoint
Proportion of adults with HIV-1 RNA ≥50 copies/mL at Week 24 (NI margin 6%) using the FDA snapshot algorithm25,26
Secondary endpoints
- Proportion of adults with HIV-1 RNA ≥50 copies/mL at Week 48 using the FDA snapshot algorithm25
- Proportion of adults with HIV-1 RNA <50 copies/mL at Weeks 24 and 4825
Extension phase
The objective of the extension phase from Week 48 to Week 72 was to evaluate the efficacy and safety of switching to BIKTARVY after additional exposure3
COVID-19 Operational Impact: Some visits between Week 48 and Week 72 were virtual due to the COVID-19 pandemic
BRAAVE 20201,2: Selected Population Characteristics
BIKTARVY (n=330)
SBR (n=165)
Median age, years (range)
49 (18-79)
49 (19-70)
69
67
31
33
96
96
2
4
5
3
American, or mixed
race that includes
Black, %
100
100
Median eGFRCG, mL/min (IQR)
110 (88-132)
107 (86-132)
Median CD4 count, cells/µL (IQR)
747 (570-922)
758 (494-969)
68
65
17
21
13
15
1
0
38
35
20
24
19
16
10
13
5
7
8
5
Frequency of Baseline Primary Resistance-Associated Substitutions1,3,4*,†
Baseline Resistance*
BIKTARVY (n=330)
SBR (n=165)
NRTI, %
13
16
1
1
9
12
5
5
NNRTI, %
21
19
10
12
5
2
PI, %
11
16
INSTI, %
2
2
1
0
- Preexisting drug resistance was assessed by cumulative historical genotype and/or proviral DNA genotypes performed retrospectively from baseline samples. Resistance to NNRTIs, PIs, and/or certain NRTIs (excluding K65R/E/N, ≥3 TAMs, and T69 insertions) was permitted; primary INSTI resistance was excluded.1,3,5
*This list is not inclusive of all RAMs observed in this analysis.3
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.6
RAM, resistance-associated mutation; TAM, thymidine-associated mutation.
References:
- Hagins D, Kumar P, Saag M, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide in Black Americans with HIV-1: a randomized Phase 3b, multicenter, open-label study. J Acquir Immune Defic Syndr. 2021;88(1):86-95.
- Hagins DP, Kumar PN, Saag M, et al. Randomized switch to B/F/TAF in African American adults with HIV. Oral abstract presented at: Conference on Retroviruses and Opportunistic Infections; March 8-11, 2020; Boston, MA. Abstract 36.
- Andreatta K, D’Antoni ML, Chang S, et al. Preexisting resistance and week 48 virologic outcomes after switching to B/F/TAF in African American adults with HIV. Oral presentation at: IDWeek 2020; October 21-25, 2020; Virtual. Oral abstract 109.
- Data on file. Gilead Sciences, Inc.
- Kumar P, Stephens JL, Wurapa AK, et al. Week 72 outcomes and COVID-19 impact from the BRAAVE 2020 study: a randomized switch to B/F/TAF in Black American adults with HIV. Poster presented at: International AIDS Society Conference on HIV Science; July 18-21, 2021; Virtual. Poster PEB161.
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
BRAAVE: Efficacy
Powerful efficacy in virologically suppressed Black Americans at 72 Weeks3,25
Switching to BIKTARVY in Black Americans was noninferior to staying on a baseline regimen at Week 24
Virologic Response in BRAAVE25,26,*

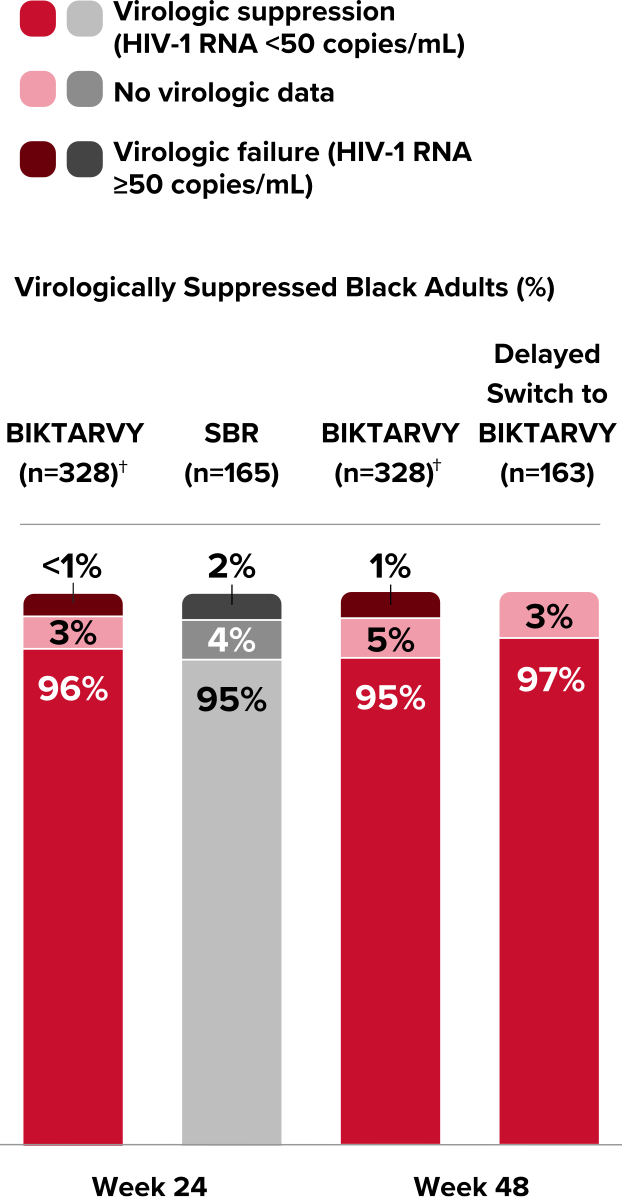
*Percentages do not total 100% due to rounding.
†Two participants were found to have baseline INSTI resistance based on historical genotype data and were excluded from the primary analysis because of this protocol violation.
Treatment outcomes between treatment groups were similar across subgroups by age and sex at Week 24.25
Subanalysis of efficacy in study participants with preexisting M184V/I resistance mutation using the FDA snapshot algorithm25

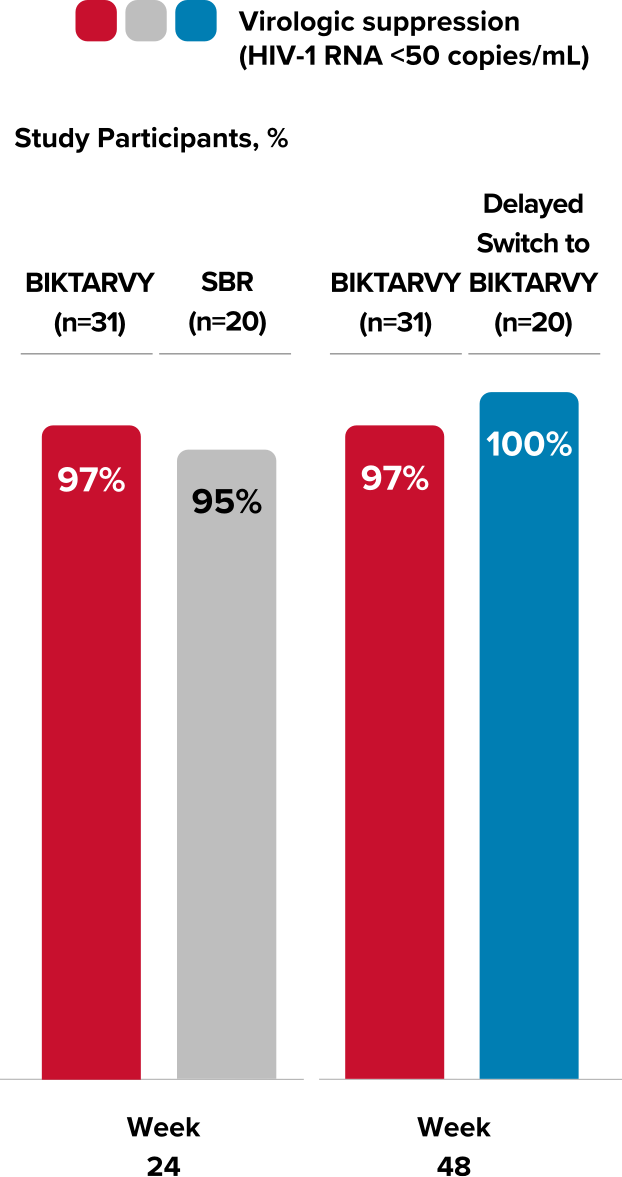
- 31 study participants randomized to BIKTARVY and 20 study participants who switched to BIKTARVY at Week 24 had M184V/I resistance mutation at baseline25
- — One participant with M184V/I taking BIKTARVY discontinued after the baseline visit and did not have virologic data at Week 24
- All study participants with M184V/I at baseline who switched to BIKTARVY and had virologic data at Week 48 remained virologically suppressed (n=50)25
Durable viral suppression at Week 723
In a 24-week extension from Week 48 through Week 72 using an M=E analysis, virologic suppression was maintained at Week 72 in 99% of participants who stayed on BIKTARVY (n=248) and 100% of participants who switched to BIKTARVY (n=127) at Week 24.3
- In an M=E analysis, participants with missing data are excluded when calculating the proportion of participants with HIV-1 RNA <50 copies/mL
BRAAVE: Resistance
Zero cases of resistance to BIKTARVY through Week 72 in virologically suppressed Black Americans3,25,28,#
Among 330 virologically suppressed adults randomized to BIKTARVY in BRAAVE through Week 24, 1 participant met criteria for resistance analysis and no treatment-emergent resistance to BIKTARVY was detected through Week 24.25
At Week 24, 320 participants continued BIKTARVY and 163 switched to BIKTARVY. Of these participants, 469 participants completed the study drug or chose to remain in the extension phase at Week 48. No additional participant was analyzed for resistance, and no treatment-emergent resistance to BIKTARVY was detected through Week 72.3,25,28
§Based on the resistance analysis population.
Post Hoc Pooled Analysis: Known or Suspected M184V/I Resistance
Switching to BIKTARVY in Virologically Suppressed People Across 6 Phase 3 Clinical Trials
Objective
Investigating the impact of known or suspected M184V/I resistance mutation on virologic response after switching to BIKTARVY29
Methods
A retrospective post hoc pooled analysis of study participant data from 6 BIKTARVY clinical trials29:
Study 1844: Virologically Suppressed Adults
BRAAVE: Virologically Suppressed Black American Adults
Study 1878: Virologically Suppressed Adults
Study 4449: Virologically Suppressed Adults ≥65 Years
Study 4030: Virologically Suppressed Adults, including those with known or suspected M184V/I resistance mutation
Study 1474: Virologically Suppressed Children & Adolescents
- Study participants were virologically suppressed (HIV-1 RNA <50 copies/mL for 3 or 6 months) on a 3-drug regimen29
- The analysis included study participants who were initially randomized to BIKTARVY and those who delayed switch to BIKTARVY after 24 or 48 weeks29
Additional Study Details
- Study 4030 and BRAAVE allowed enrollment of participants with documented M184V/I resistance mutation29
- Studies 1844, 1878, 4449, and 1474 excluded those with known or suspected M184V/I resistance mutation at screening29
- Composite baseline resistance was derived from cumulative historical and/or proviral DNA genotypic data obtained at or after enrollment in trials29
- Some study participants had exclusionary drug resistance substitutions detected after study drugs were initiated29
- All participants retrospectively found to have preexisting resistance to any component of BIKTARVY were allowed to continue and were included in efficacy analyses29
182 virologically suppressed BIKTARVY-treated study participants across 6 clinical trials with preexisting M184V/I resistance mutation, including29,*:
Known or suspected preexisting M184V/I resistance allowed at enrollment†
Study 4030
Virologically Suppressed Adults29
BRAAVE
Virologically Suppressed Black American Adults29
n=47
n=50
Known or suspected preexisting M184V/I resistance excluded at enrollment and retrospectively found‡
Study 1844
Virologically Suppressed Adults29
Study 1878
Virologically Suppressed Adults29
n=17
n=62
Study 1474
Virologically Suppressed Children &
Adolescents29
Study 4449
Virologically Suppressed Adults Aged ≥65 Years29
n=3
n=3
Frequency of Baseline M184V/I Resistance Mutation in Study Participants Who Switched to BIKTARVY29
M184V/I Resistance Mutation
Participants with baseline PR/RT genotype
available
(n=1825), n (%)
182 (10%)
38 (4%)
167 (10%)
Includes study participants with available PR/RT data and at least one post-switch on treatment HIV-1 RNA.
*Based on study participants with available baseline PR/RT genotype who switched to BIKTARVY at baseline or after 24 or 48 weeks (n=182/1825).
†Documented or suspected resistance to NRTls, NNRTls, or PIs was permitted in Study 4030. History of resistance to NRTls (excluding 3 or more TAMS, T69 insertions, or K65R/E/N in RT), NNRTls, or PIs was permitted in BRAAVE.
‡Historical genotypes were assessed at screening, and documented resistance to study drugs led to exclusion. Retrospective deep sequencing analysis was later attempted on baseline samples from all BIKTARVY-treated participants.
Preexisting resistance at baseline in BIKTARVY-treated study participants
Frequency of Baseline Resistance-Associated Substitutions29,§
Baseline Genotype, n (%)
Pooled BIKTARVY Study Participants
PR/RT (n=1825) and IN (n=1731)
NRTI||
288 (16%)
182 (10%)
18 (1%)
167 (9%)
NNRTI¶
397 (22%)
205 (11%)
V179L, Y181C/I/V, Y188L, H221Y,
F227C, and/or M230I/L
170 (9%)
PI#
201 (11%)
Primary INSTI**
30 (2%)
Preexisting drug resistance was assessed by historical genotypes and/or baseline proviral DNA genotyping.
§BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.
||Nucleoside reverse transcriptase inhibitor resistance (NRTI-R) substitutions were K65R/E/N, T69 insertions, K70E, L74V/1, Y11SF, Q151M, M184V/I, and thymidine analog mutations (M41L, D67N, K70R, L210W, T215F/Y, and K219E/N/Q/R) in reverse transcriptase.
¶Non-nucleoside reverse transcriptase inhibitor resistance (NNRTI-R) substitutions were L1OOI, K1O1E/P, K103N/S, V106M/A, V1081, E138A/G/K/Q/R, V179L, Y181C/I/V, Y188C/H/L, G190A/E/Q/S, H221Y, P225H, F227C, and M230L/I in reverse transcriptase.
#Protease inhibitor resistance (PI-R) substitutions were D30N, V32I, M46I/L, 147V/A, G48V, I5OV/L, 154M/L, Q58E, T74P, L76V, V82A/F/L/S/T, N83D, I84V, N88S, and L90M in protease.
**Primary integrase strand transfer inhibitor resistance (INSTI-R) substitutions were T66I/A/K, E92Q/G, F121Y, Y143R/H/C, S147G, Q148H/K/R, N155H/S, and R263K in integrase.
Risk Factors Associated with Preexisting M184V/I Resistance Found in Pooled Analysis 1,*
M184V/I Present
Odds Ratio
(95% CI)Presence of NRTI resistance (other than M184V/I)
4.64
(3.10-6.95)Presence of NNRTI resistance
3.29
(2.30-4.70)Presence of PI resistance
2.49
(1.59-3.91)Black race (vs non-Black)
2.10
(1.37-3.20)Hispanic/Latino ethnicity (vs not Hispanic/Latino)
1.67
(1.03-2.71)HIV status: symptomatic or AIDS (vs asymptomatic)
1.66
(1.08-2.57)CD4 <500 cells/μl
(vs ≥500 cells/μl)
1.52
(1.04-2.22)Number of prior third agents
1.17
(1.02-1.34)Time since ART start (per year)
1.10
(1.07-1.12)The multivariate regression model includes all study participants in the BIKTARVY treatment arm and comparator treatment arms.
*Multivariate logistic regression model included baseline resistance and characteristics as potential risk factors and was adjusted for study-specific effects. Factors associated with preexisting Ml84V/I only included data from adult trials (excluded 1474). BRAAVE (Study 4580) included only study participants who self-identified as Black race, which could have biased the analysis of Ml84V/I prevalence and risk factors. Other enrollment criteria, including age and baseline regimen, may also have affected the analysis of M184V/I risk factors; however, study-specific effects were included in the multivariate model. Furthermore, potentially incomplete ART histories could have affected our model's accuracy.
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CD4, cluster of differentiation 4; NNRTI, non-nucleoside reverse transcriptase inhibitor: NRTI, nucleoside reverse transcriptase inhibitor; Pl, protease inhibitor.
References:
- Sax PE, Andreatta K, Molina J-M, et al. High efficacy of switching to bictegravir/emtricitabine/tenofovir alafenamide in people with suppressed HIV and preexisting M184V/I. AIDS. 2022;36(11):1511-1520.
Viral Suppression in BIKTARVY-Treated Study Participants with Known or Suspected Preexisting M184V/I Resistance Mutation
Virologic Response Using M=E Analysis29
Methods
The analysis included study participants who were initially randomized to BIKTARVY and those who delayed switch to BIKTARVY after 24 or 48 weeks.
In an M=E analysis, study participants with missing data are excluded when calculating the proportion of participants with HIV-1 RNA <50 copies/mL

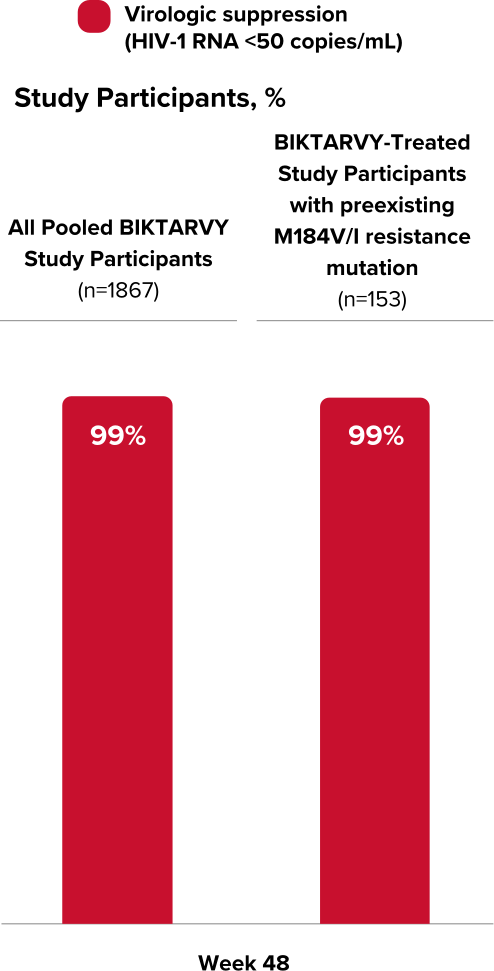
Retrospective Post Hoc Pooled Analysis: Known or Suspected Preexisting M184V/I Resistance
Among 2044 virologically suppressed people included in the retrospective post hoc pooled analysis29,†:
- 7 met the criteria for resistance analysis. No treatment-emergent resistance was detected.‡
*Based on the resistance analysis population or final resistance analysis population, as applicable.
†Includes all participants in the overall missing=excluded analysis population who switched to BIKTARVY in the post hoc pooled analysis.
‡Resistance outcomes are presented through each respective study's treatment duration. Due to the varying lengths of extension phases in each study, the duration of each study varied.
Virologic Response Using the FDA Snapshot Algorithm12
Methods
- In another analysis, efficacy was evaluated in study participants with available baseline RT genotype data using the FDA snapshot algorithm (n=1225)
- The analysis included only study participants initially randomized to BIKTARVY from Studies 1844, 1878, BRAAVE, 4030, 4449, and 1474 cohort 1
Outcomes
- Proportion of study participants with HIV-1 RNA ≥50 copies/mL at Week 48 as determined by the US FDA-defined snapshot algorithm

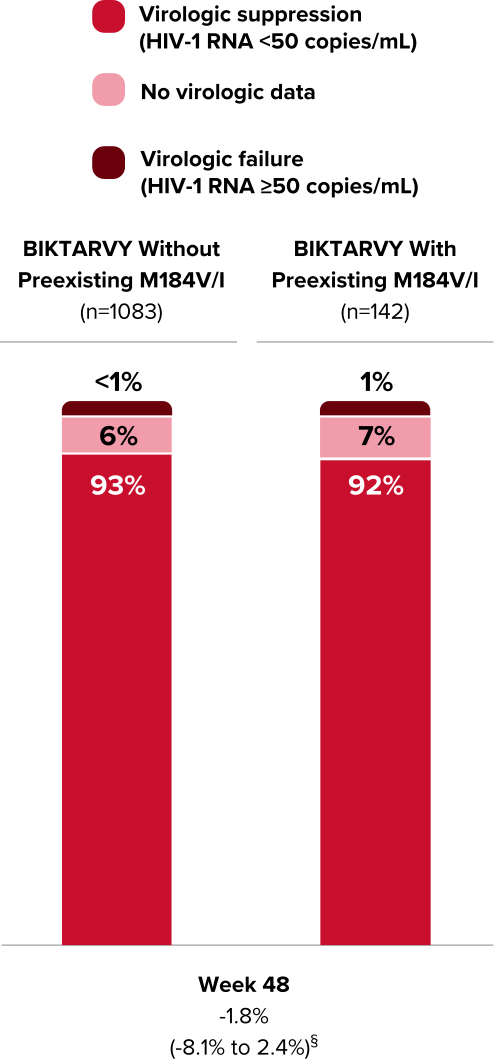
§Treatment difference in HIV-1 RNA <50 copies/mL (95% confidence interval)


People With HIV Aged 50 and Older
Explore BIKTARVY clinical trial data.
Please see full Prescribing Information for BIKTARVY® and DESCOVY® , including BOXED WARNINGS.
3TC, lamivudine; ABC, abacavir; ATV, atazanavir; BIC, bictegravir; C, cobicistat; DRV, darunavir; DTG, dolutegravir; E/EVG, elvitegravir; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); F, emtricitabine; FDA, US Food and Drug Administration; FTC, emtricitabine; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; M=E, missing=excluded; M=F, missing=failure; NI, noninferiority; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OLE, open-label extension; PK, pharmacokinetics; QD, once daily; RNA, ribonucleic acid; RPV, rilpivirine; RT, reverse transcriptase; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
References:
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
- Kityo C, Hagins D, Koenig E, et al. Longer-term (96-week) efficacy and safety of switching to bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in women. Oral presentation at: International AIDS Society Conference on HIV Science 2019; July 21-24, 2019; Mexico City, Mexico. Abstract MOAB0106.
- Kumar P, Stephens JL, Wurapa AK, et al. Week 72 outcomes and COVID-19 impact from the BRAAVE 2020 study: a randomized switch to B/F/TAF in Black American adults with HIV. Poster presented at: International AIDS Society Conference on HIV Science; July 18-21, 2021; Virtual. Poster PEB161.
- Molina J-M, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, Phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357-e365.
- Brar I, Ruane P, Berhe M, et al. Efficacy and safety of switch to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir/abacavir/lamivudine: Results from an open-label extension of a phase 3 randomized, double-blind, multicenter, active-controlled, non-inferiority study. Medicine. 2025;104(8):e41482.
- Safety and efficacy of switching from dolutegravir and ABC/3TC or ABC/DTG/3TC to B/F/TAF in HIV-1 infected adults who are virologically suppressed. ClinicalTrials.gov identifier: NCT02603120. Updated November 12, 2020. Accessed January 9, 2023. https://clinicaltrials.gov/ct2/show/NCT02603120
- Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347-e356.
- Rockstroh J, Molina J-M, Post F, et al. Long-term follow-up after a switch to bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) from a boosted protease inhibitor-based regimen. Poster presented at: HIV Drug Therapy Glasgow 2020; October 5-8, 2020; Virtual. Poster P036.
- Study to evaluate the safety and efficacy of switching from regimens consisting of boosted atazanavir or darunavir plus either emtricitabine/tenofovir or abacavir/lamivudine to bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed HIV-1 infected adults. ClinicalTrials.gov identifier: NCT02603107. Updated December 29, 2020. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT02603107
- Andreatta K, William M, Martin R, et al. Resistance analyses of bictegravir/emtricitabine/tenofovir alafenamide switch studies. Poster presented at: Conference on Retroviruses and Opportunistic Infections; March 4-7, 2018; Boston, MA. Poster 506.
- Andreatta K, Chang S, Delaney M, et al. Long-term efficacy among participants switched to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir/abacavir/lamivudine with preexisting resistance and viral blips. Poster presented at: International AIDS Society Conference on HIV Science; July 18-21, 2021; Virtual. Poster PEB172.
- Data on file. Gilead Sciences, Inc.
- Sax PE, Rockstroh JK, Luetkemeyer AF, et al; GS-US-380-4030 Investigators. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis. 2021;73(2):e485-e493.
- Switching to a fixed dose combination of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in human immunodeficiency virus type 1 (HIV-1) infected adults who are virologically suppressed. ClinicalTrials.gov identifier: NCT03110380. Updated January 11, 2021. Accessed November 17, 2023. https://clinicaltrials.gov/study/NCT03110380
- Kityo C, Hagins D, Koenig E, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, Phase 3, noninferiority trial. J Acquir Immune Defic Syndr. 2019;82(3):321-328.
- Safety and efficacy of switching to a FDC of B/F/TAF from E/C/F/TAF, E/C/F/TDF, or ATV+RTV+FTC/TDF in virologically suppressed HIV-1 infected women. ClinicalTrials.gov identifier: NCT02652624. Updated March 4, 2020. Accessed January 9, 2023. https://clinicaltrials.gov/ct2/show/NCT02652624
- Maggiolo F, Rizzardini G, Molina J-M, et al. Bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people with HIV aged ≥65 years: week 48 results of a Phase 3b, open-label trial. Infect Dis Ther. 2021;10(2):775-788.
- Maggiolo F, Rizzardini G, Molina JM, et al. Bictegravir/emtricitabine/tenofovir alafenamide in older individuals with HIV: Results of a 96-week, phase 3b, open-label, switch trial in virologically suppressed people ≥65 years of age. HIV Med. 2023;24(1):27-36.
- Study to evaluate switching from an E/C/F/TAF fixed-dose combination (FDC) regimen or a TDF containing regimen to B/F/TAF FDC in human immunodeficiency virus-1 (HIV-1) infected participants aged > 65 years. ClinicalTrials.gov identifier: NCT03405935. Updated December 1, 2020. Accessed January 9, 2023. https://clinicaltrials.gov/ct2/show/NCT03405935
- Maggiolo F, Rizzardini G, Molina J-M, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in adults aged ≥65 years: week 72 results from a Phase 3b, open-label trial (GS-US-380-4449). Poster presented at: HIV Drug Therapy Glasgow. 2020; October 5-8, 2020; Glasgow, UK. Poster P038.
- Gaur AH, Cotton MF, Rodriguez CA, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide in adolescents and children with HIV: week 48 results of a single-arm, open-label, multicentre, phase 2/3 trial. Lancet Child Adolesc Health. 2021;5(9):642-651.
- Natukunda E, Rodriguez CA, McGrath EJ, et al. B/F/TAF in virologically suppressed adolescents and children: two-year outcomes in 6 to <18 year olds and six-month outcomes in toddlers. Abstract presented at: International Workshop on HIV & Pediatrics. July 16-17, 2021; Virtual. Abstract 2.
- Study of bictegravir/emtricitabine/tenofovir alafenamide fixed dose combination in adolescents and children with human immunodeficiency virus-1. ClinicalTrials.gov identifier: NCT02881320. Updated August 8, 2023. Accessed August 31, 2023. https://clinicaltrials.gov/study/NCT02881320
- Rodriguez CA, Strehlau R, Chokephaibulkit K, et al. One year outcome of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in virologically suppressed children ≥ 2 years weighing 14 to < 25 kg. Oral presentation at: International Workshop on HIV & Pediatrics 2022; July 27-28, 2022; Montreal, Quebec, Canada. Oral 2.
- Hagins D, Kumar P, Saag M, et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide in Black Americans with HIV-1: a randomized Phase 3b, multicenter, open-label study. J Acquir Immune Defic Syndr. 2021;88(1):86-95.
- Hagins DP, Kumar PN, Saag M, et al. Randomized switch to B/F/TAF in African American adults with HIV. Oral abstract presented at: Conference on Retroviruses and Opportunistic Infections; March 8-11, 2020; Boston, MA. Abstract 36.
- Study to evaluate switching from a regimen of two nucleos(t)ide reverse transcriptase inhibitors (NRTI) plus a third agent to a fixed dose combination (FDC) of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF), in virologically-suppressed, HIV-1 infected African American participants (BRAAVE 2020). ClinicalTrials.gov identifier: NCT03631732. Updated September 5, 2021. Accessed January 9, 2023. https://clinicaltrials.gov/ct2/show/NCT03631732
- Andreatta K, D’Antoni ML, Chang S, et al. High efficacy of bictegravir/emtricitabine/tenofovir alafenamide in African-American adults with HIV including those with preexisting resistance, viral blips, and suboptimal adherence. Presented at: IDWeek 2021; September 29-October 3, 2021; Virtual. Poster 629.
- Sax PE, Andreatta K, Molina J-M, et al. High efficacy of switching to bictegravir/emtricitabine/tenofovir alafenamide in people with suppressed HIV and preexisting M184V/I. AIDS. 2022;36(11):1511-1520.