31 (18-71)
Treatment-Naïve Patients
Study 14891,2
BIKTARVY (n=314)
ABC/DTG/
3TC (n=315)
Median age, years (range)
32 (18-68)
91
90
9
10
57
57
36
36
2
3
Hispanic/Latino, %
23
21
Median HIV-1 RNA, log10
copies/mL (IQR)
4.42 (4.03-4.87)
4.51 (4.04-4.87)
HIV-RNA >100,000 copies/mL, %
17
16
Median CD4 cell count,
cells/µL (IQR)
443 (299-590)
450 (324-608)
CD4 count <200 cells/µL, %
11
10
Asymptomatic HIV, %
91
91
Median eGFRCG, mL/min (IQR)
126 (107.7-146.3)
123 (107.0-144.3)
Retrospective Analysis of Preexisting Resistance at Baseline in Studies 1489 & 14903,*,†
BIKTARVY Arms From Studies 1489 & 1490 N‡=632 (IN) N‡=634 (PR/RT)
INSTI, %
1.1
NRTI, %
3.3
NNRTI, %
12.9
PI, %
3.0
- Study participants with preexisting resistance substitutions causing reduced susceptibility to FTC, TAF, ABC, and 3TC were excluded at screening. Retrospective deep sequencing analysis was later attempted on baseline samples from all BIKTARVY-treated participants.
*This list is not inclusive of all RAMs observed in this analysis.3
†BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.4
‡Retrospective deep sequencing data were combined with the population sequencing data from screening.
3TC, lamivudine; ABC, abacavir; CD4, cluster of differentiation 4; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); FTC, emtricitabine, IN, integrase; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PR, protease; RAM, resistance-associated mutation; RT, reverse transcriptase; TAM, thymidine-associated mutation.
References:
- Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355-e363.
- Orkin C, DeJesus E, Sax PE, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, Phase 3, non-inferiority trials. Lancet HIV. 2020;7(6):e389-e400.
- Acosta RK, Chen GQ, Chang S, et al. Three-year study of pre-existing drug resistance substitutions and efficacy of bictegravir/emtricitabine/tenofovir alafenamide in HIV-1 treatment-naïve participants. J Antimicrob Chemother. 2021;76(8):2153-2157.
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
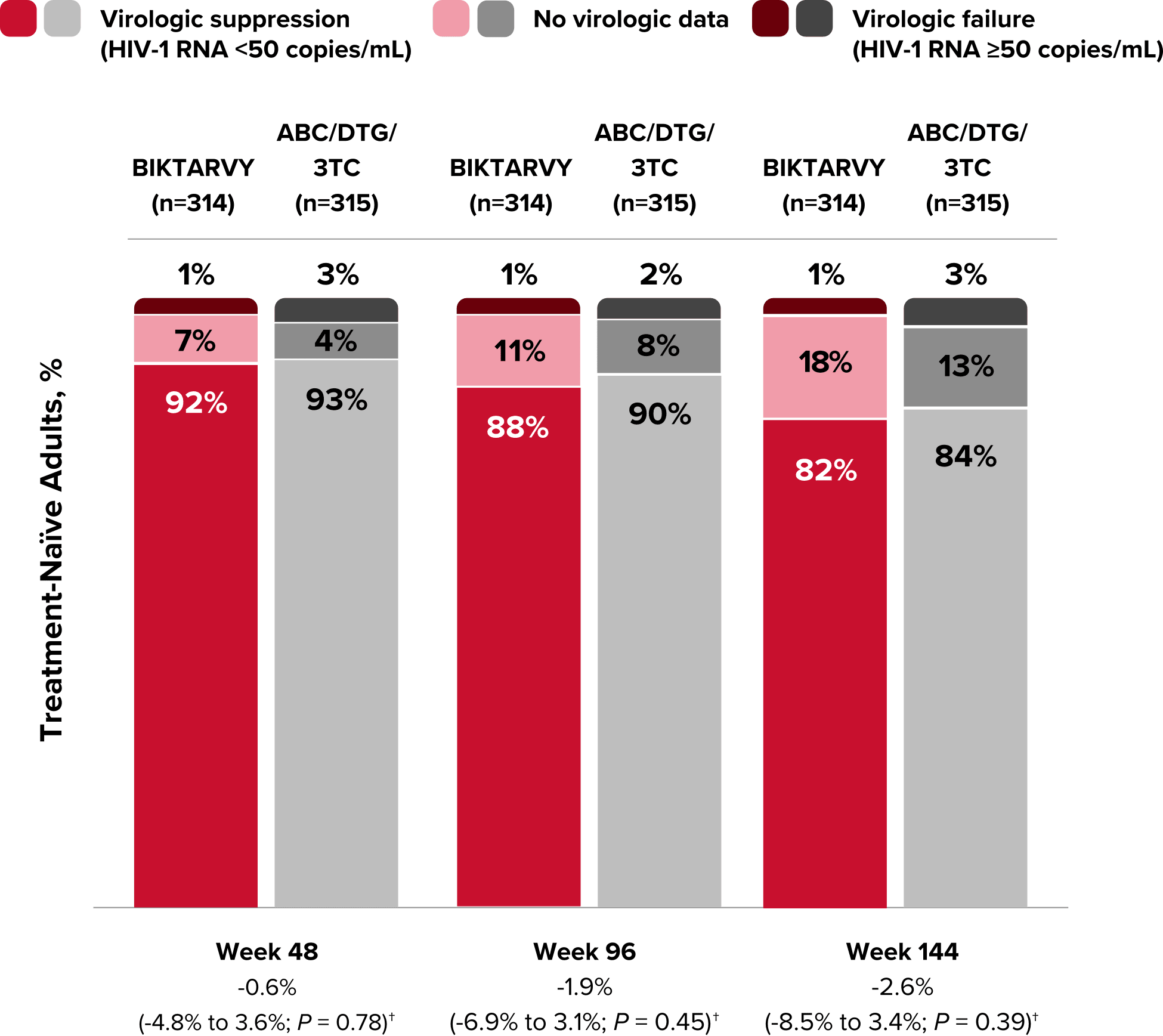
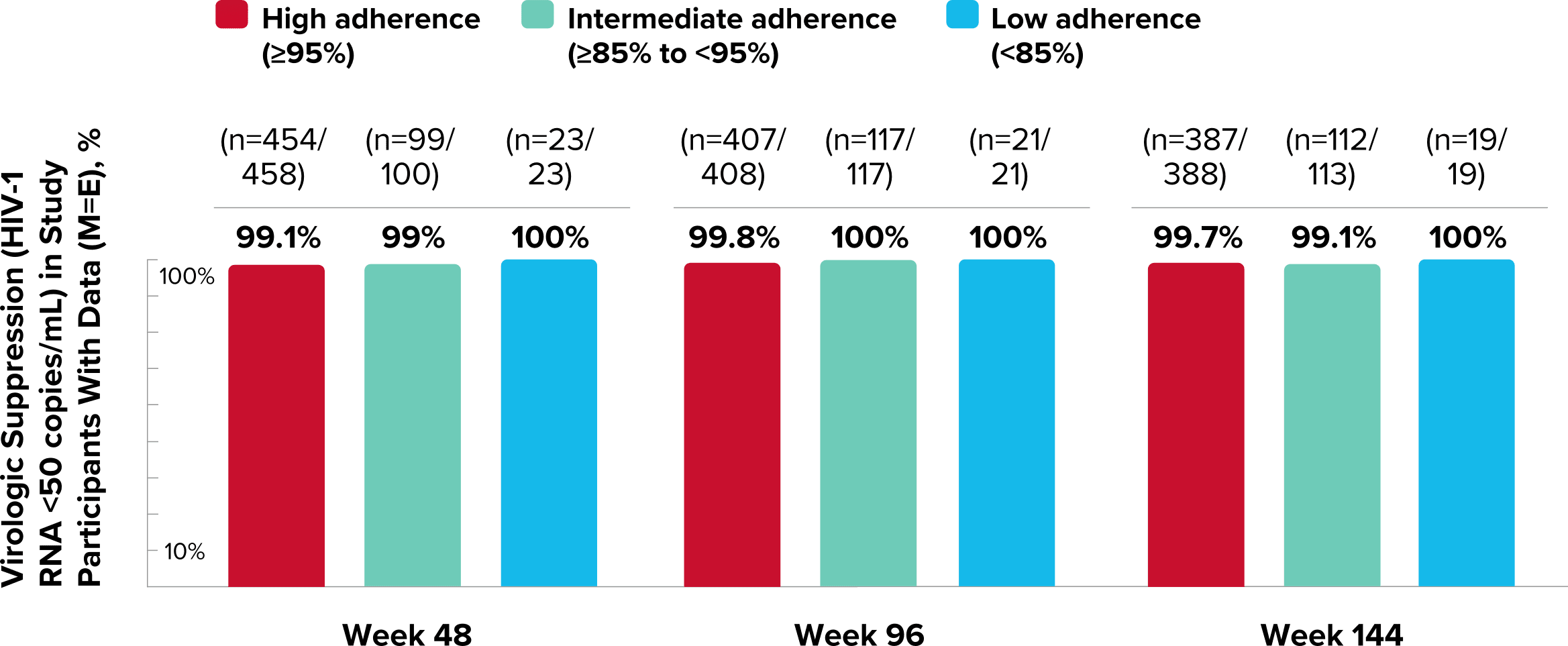
Study 1489 Virologic Outcomes1-4,*
Week 48
Week 96
Week 144
BIKTARVY
(n=314)
ABC/
DTG/3TC
(n=315)
BIKTARVY
(n=314)
ABC/
DTG/3TC
(n=315)
BIKTARVY†
(n=314)
ABC/
DTG/3TC
(n=315)
HIV-1 RNA <50
copies/mL
92%
93%
88%
90%
82%
84%
-0.6%
(-4.8% to 3.6%)
-1.9%
(-6.9% to 3.1%)
-2.6%
(-8.5% to 3.4%)
HIV-1 RNA ≥50
copies/mL‡
1%
3%
1%
2%
1%
3%
No virologic data
7%
4%
11%
8%
18%
13%
0%
1%
<1%
2%
1%
2%
5%
3%
10%
5%
16%
11%
2%
<1%
1%
1%
1%
<1%
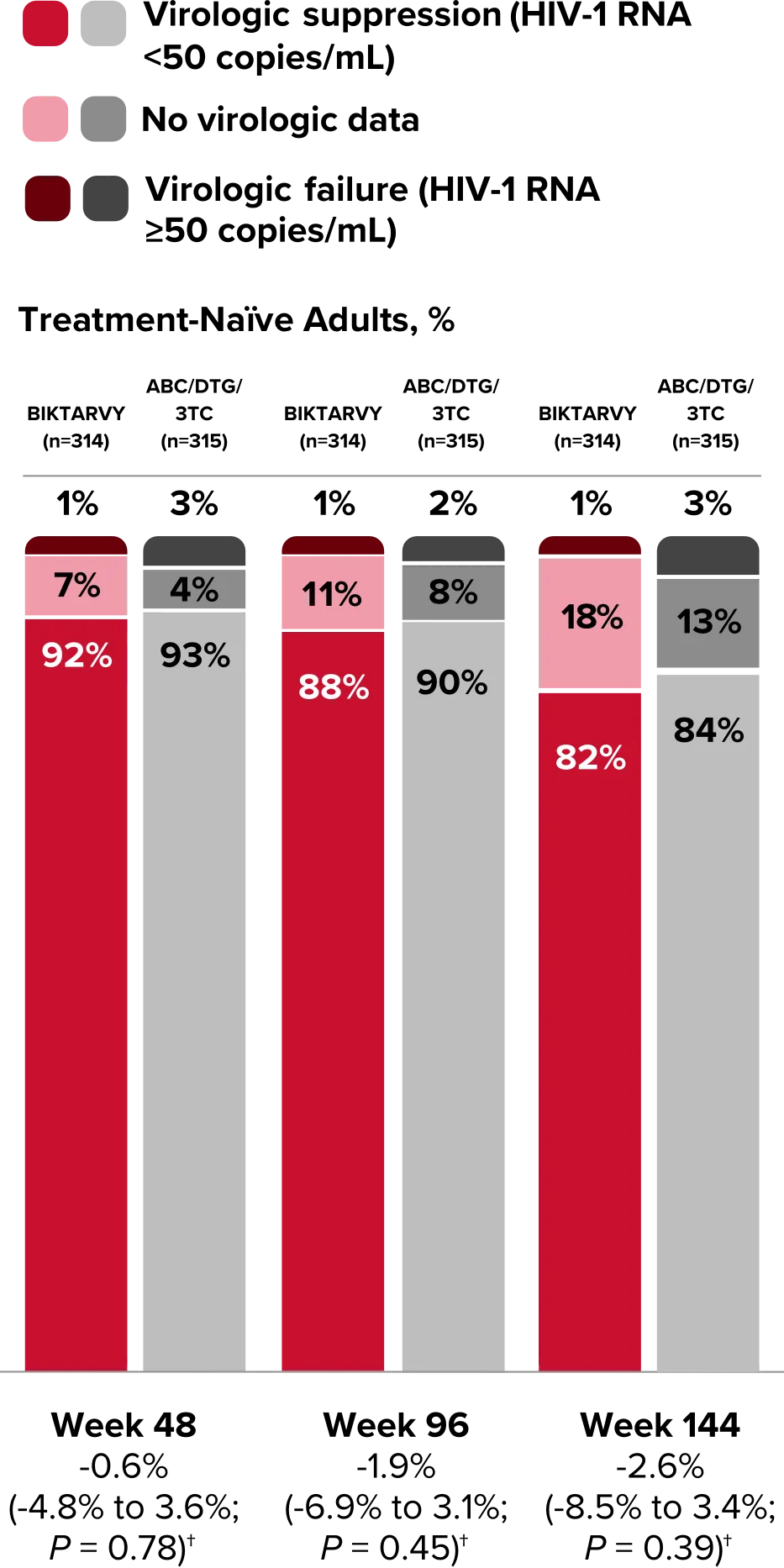
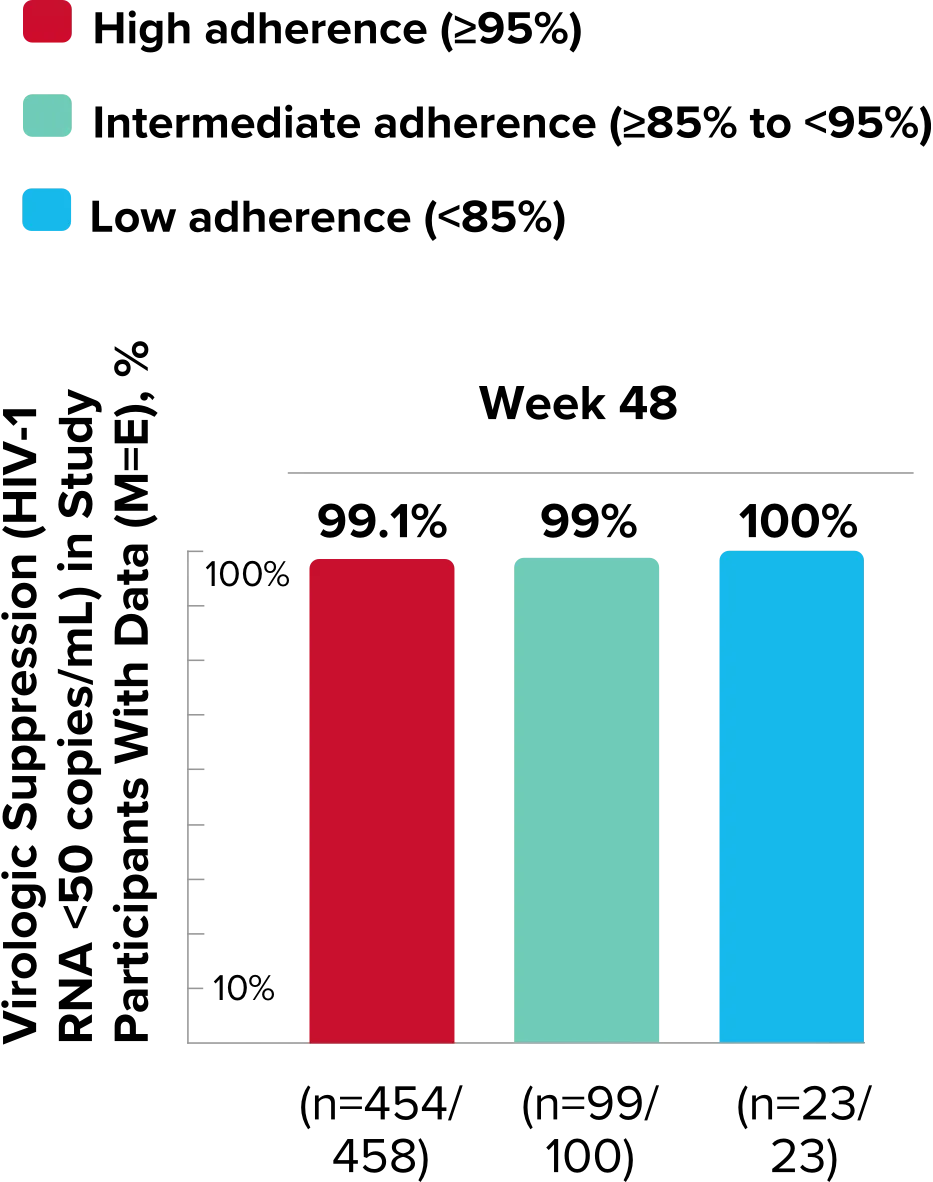
Study 1489 Virologic Outcomes1-4,*
Week 48
BIKTARVY
(n=314)
ABC/
DTG/3TC (n=315)
HIV-1 RNA <50
copies/mL
92%
93%
(95% CI) BIKTARVY vs comparator
-0.6%
(-4.8% to 3.6%)
HIV-1 RNA ≥50
copies/mL‡
1%
3%
No virologic data
7%
4%
0%
1%
RNA <50 copies/mL||
5%
3%
window but on
study drug
2%
<1%
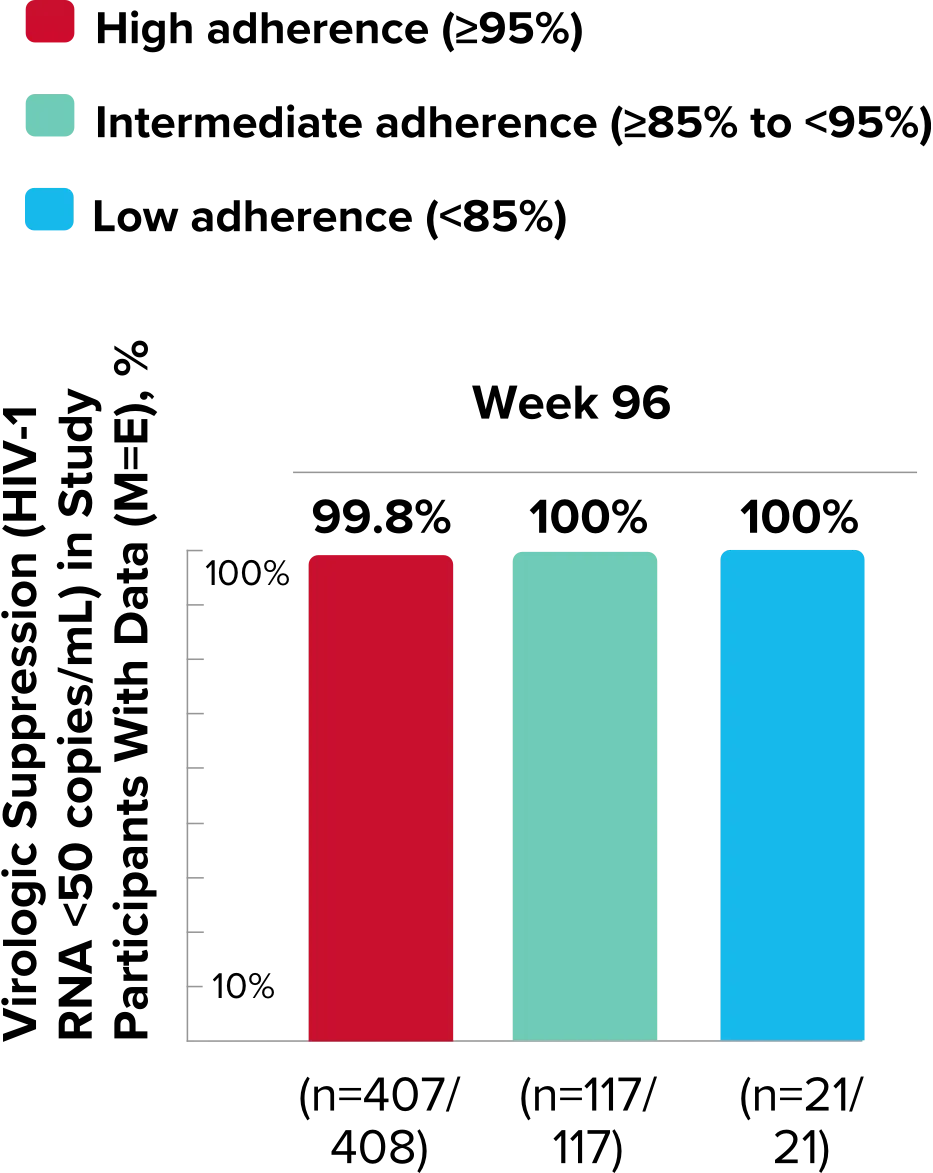
Study 1489 Virologic Outcomes1-4,*
Week 96
BIKTARVY
(n=314)
ABC/
DTG/3TC (n=315)
HIV-1 RNA <50
copies/mL
88%
90%
-1.9%
(-6.9% to 3.1%)
HIV-1 RNA ≥50
copies/mL‡
1%
2%
No virologic data
11%
8%
<1%
2%
10%
5%
1%
1%
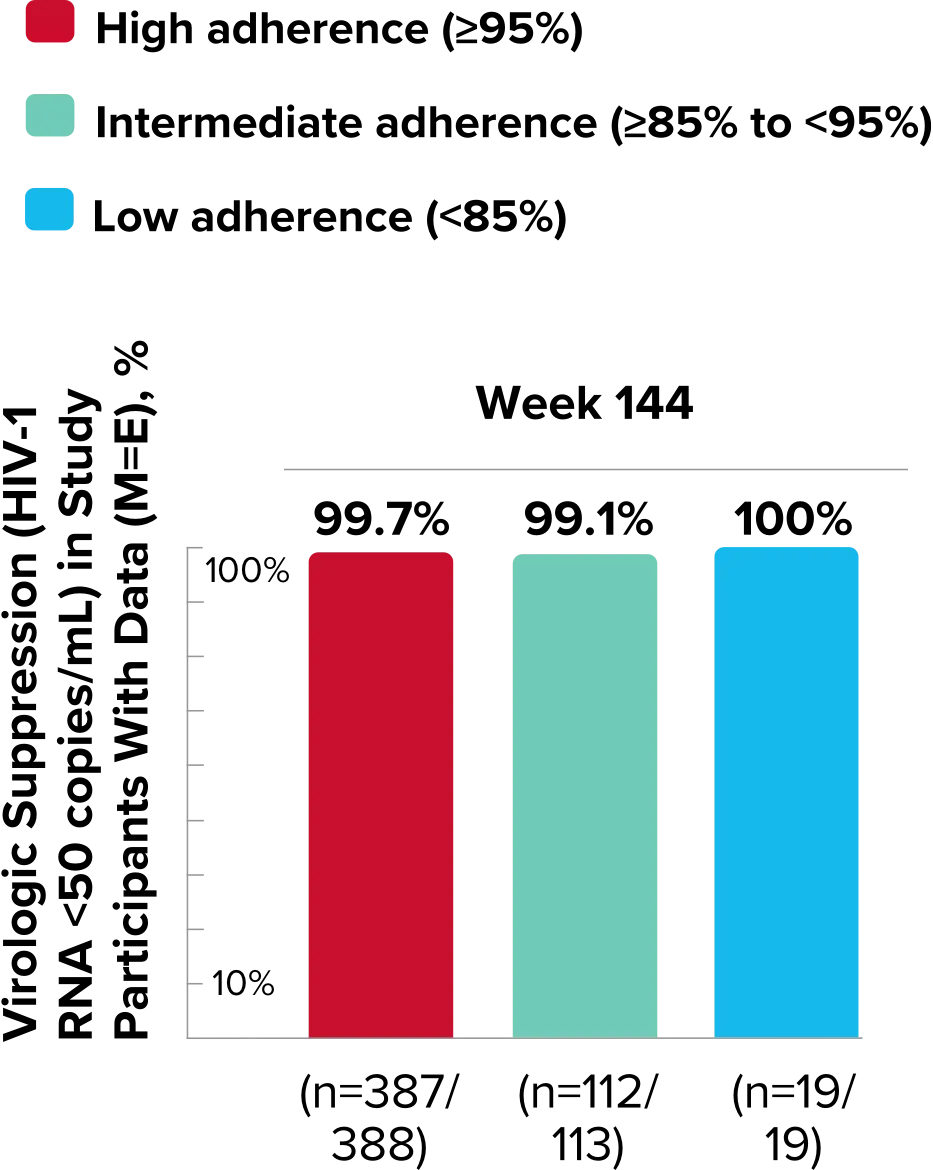
Study 1489 Virologic Outcomes1-4,*
Week 144
BIKTARVY†
(n=314)
ABC/
DTG/3TC (n=315)
HIV-1 RNA <50
copies/mL
82%
84%
-2.6%
(-8.5% to 3.4%)
HIV-1 RNA ≥50
copies/mL‡
1%
3%
No virologic data
18%
13%
1%
2%
16%
11%
1%
<1%
*Week 48 window was between Day 295 and 378 (inclusive); Week 96 window was between Day 631 and 714 (inclusive); Week 144 window was between Day 967 and 1050 (inclusive).
†Percentages do not total 100% due to rounding.
‡Includes participants who had ≥50 copies/mL in the Week 48, 96, or 144 window; participants who discontinued early due to lack or loss of efficacy; and participants who discontinued for reasons other than an adverse event (AE), death, or lack or loss of efficacy and at the time of discontinuation had a viral value of ≥50 copies/mL.
§Includes participants who discontinued due to AE or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
||Includes participants who discontinued for reasons other than an AE, death, or lack or loss of efficacy; eg, withdrew consent, loss to follow-up, etc.
CI, confidence interval.
References:
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
- Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, Phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063-2072.
- Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355-e363.
- Orkin C, DeJesus E, Sax PE, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, Phase 3, non-inferiority trials. Lancet HIV. 2020;7(6):e389-e400.
Study 14901,2
BIKTARVY(n=320)
DTG+FTC/
TAF(n=325)
Median age, years (range)
33 (18-71)
34 (18-77)
88*
89
13*
11
57
60
30
31
2
3
Hispanic/Latino, %
26
25
Median HIV-1 RNA, log10
copies/mL (IQR)
4.43 (3.95-4.90)
4.45 (4.03-4.84)
HIV-RNA >100,000 copies/mL, %
21
17
Median CD4 cell count,
cells/µL (IQR)
440 (289-591)
441 (297-597)
CD4 count <200 cells/µL, %
14
10
Asymptomatic HIV, %
89
89
Median eGFRCG, mL/min (IQR)
120.4 (100.8-141.8)
120.6 (102.8-145.1)
Retrospective Analysis of Preexisting Resistance at Baseline in Studies 1489 & 14903,†,‡
BIKTARVY Arms From Studies 1489 & 1490 N§=632 (IN) N§=634 (PR/RT)
INSTI, %
1.1
NRTI, %
3.3
NNRTI, %
12.9
PI, %
3.0
- Study participants with preexisting resistance substitutions causing reduced susceptibility to FTC, TAF, ABC, and 3TC were excluded at screening. Retrospective deep sequencing analysis was later attempted on baseline samples from all BIKTARVY-treated participants.
*Percentages do not total 100% due to rounding.
†This list is not inclusive of all RAMs observed in this analysis.3
‡BIKTARVY is not indicated for patients with known or suspected substitutions associated with resistance to bictegravir or tenofovir.4
§Retrospective deep sequencing data were combined with the population sequencing data from screening.
3TC, lamivudine; ABC, abacavir; CD4, cluster of differentiation 4; eGFRCG, estimated glomerular filtration rate (Cockcroft-Gault); FTC, emtricitabine; IN, integrase; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PR, protease; RAM, resistance-associated mutation; RT, reverse transcriptase; TAM, thymidine-associated mutation.
References:
- Stellbrink H-J, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364-e372.
- Orkin C, DeJesus E, Sax PE, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, Phase 3, non-inferiority trials. Lancet HIV. 2020;7(6):e389-e400.
- Acosta RK, Chen GQ, Chang S, et al. Three-year study of pre-existing drug resistance substitutions and efficacy of bictegravir/emtricitabine/tenofovir alafenamide in HIV-1 treatment-naïve participants. J Antimicrob Chemother. 2021;76(8):2153-2157.
- BIKTARVY. Prescribing Information. Gilead Sciences, Inc.; 2025.
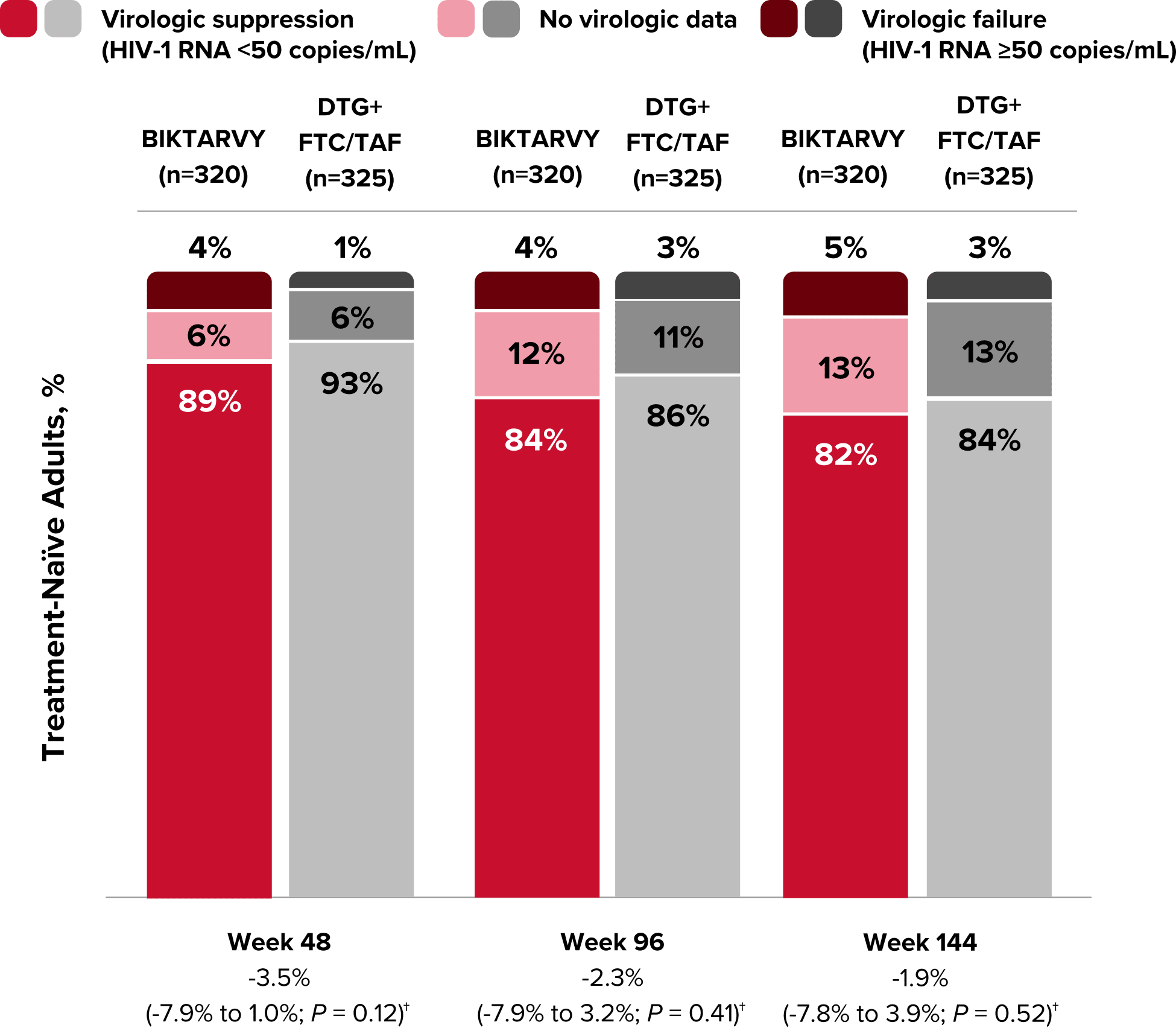
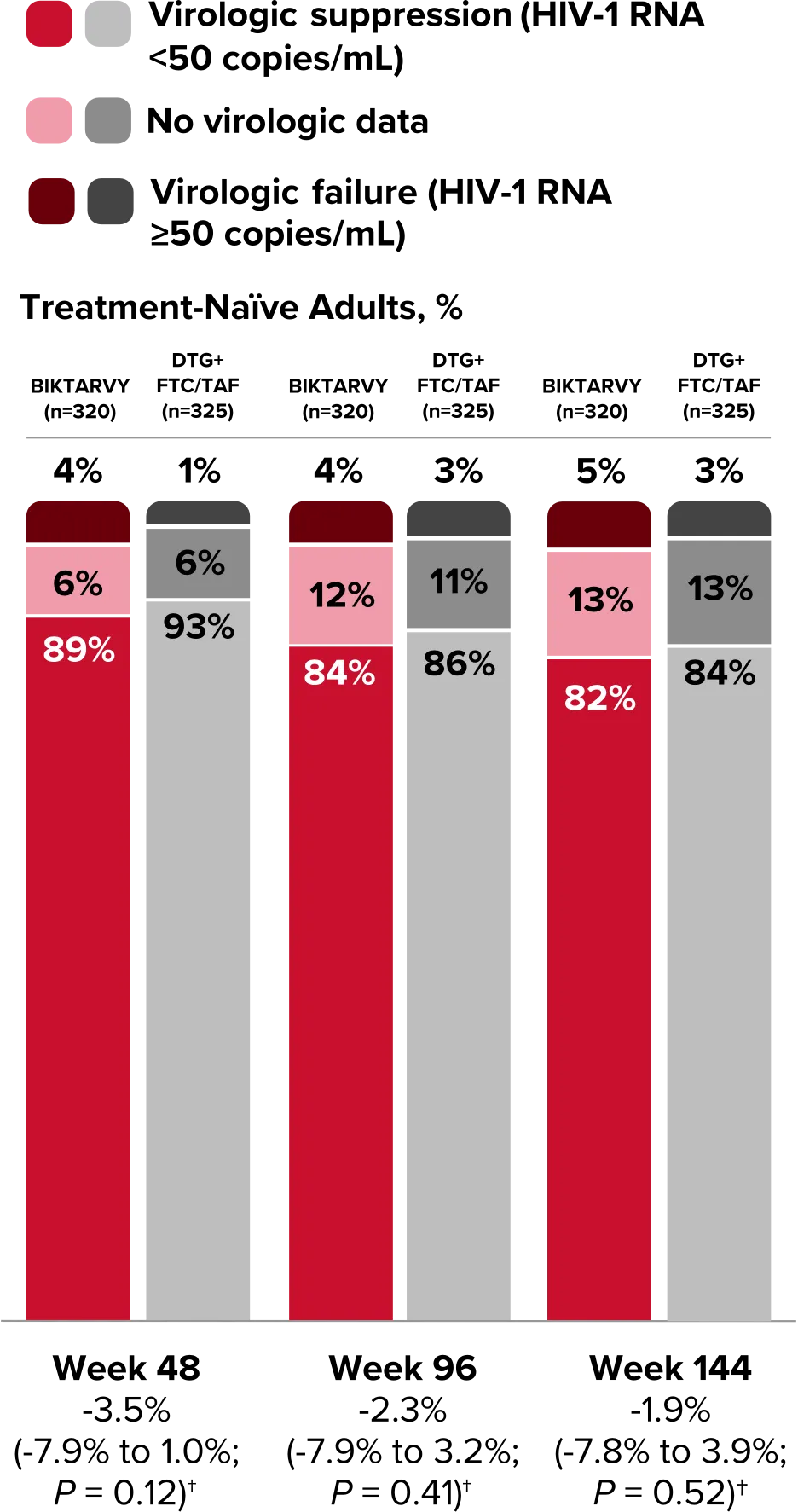
Study 1490 Virologic Outcomes1-4,*
Week 48
Week 96
Week 144
BIKTARVY
(n=320)
DTG/
FTC/TAF
(n=325)
BIKTARVY
(n=320)
DTG/
FTC/TAF
(n=325)
BIKTARVY
(n=320)
DTG/
FTC/TAF
(n=325)
HIV-1 RNA <50
copies/mL
89%
93%
84%
86%
82%
84%
-3.5%
(-7.9% to 1.0%)
-2.3%
(-7.9% to 3.2%)
-1.9%
(-7.8% to 3.9%)
HIV-1 RNA ≥50
copies/mL‡
4%
1%
4%
3%
5%
3%
No virologic data
6%
6%
12%
11%
13%
13%
1%
1%
3%
2%
3%
3%
3%
4%
8%
7%
11%
9%
2%
1%
1%
1%
0%
1%
Study 1490 Virologic Outcomes1-4,*
Week 48
BIKTARVY
(n=320)
DTG/
FTC/TAF (n=325)
HIV-1 RNA <50
copies/mL
89%
93%
(95% CI) BIKTARVY vs comparator
-3.5%
(-7.9% to 1.0%)
HIV-1 RNA ≥50
copies/mL‡
4%
1%
No virologic data
6%
6%
1%
1%
RNA <50 copies/mL||
3%
4%
window but on
study drug
2%
1%
Study 1490 Virologic Outcomes1-4,*
Week 96
BIKTARVY
(n=320)
DTG/
FTC/TAF(n=325)
84%
86%
-2.3%
(-7.9% to 3.2%)
4%
3%
12%
11%
3%
2%
8%
7%
1%
1%
Study 1490 Virologic Outcomes1-4,*
Week 144
BIKTARVY†
(n=320)
DTG/
FTC/TAF(n=325)
82%
84%
-1.9%
(-7.8% to 3.9%)
5%
3%
13%
13%
3%
3%
11%
9%
0%
1%
*Week 48 window was between Day 295 and 378 (inclusive); Week 96 window was between Day 631 and 714 (inclusive); Week 144 window was between Day 967 and 1050 (inclusive).
†Includes participants who had ≥50 copies/mL in the Week 48, 96, or 144 window; participants who discontinued early due to lack or loss of efficacy; and participants who discontinued for reasons other than an adverse event (AE), death, or lack or loss of efficacy and at the time of discontinuation had a viral value of ≥50 copies/mL.
‡Includes participants who discontinued due to AE or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
§Includes participants who discontinued for reasons other than an AE, death, or lack or loss of efficacy; eg, withdrew consent, loss to follow-up, etc.
CI, confidence interval.
References:
- BIKTARVY. Prescribing information. Gilead Sciences, Inc.; 2025.
- Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073-2082.
- Stellbrink H-J, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364-e372.
- Orkin C, DeJesus E, Sax PE, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, Phase 3, non-inferiority trials. Lancet HIV. 2020;7(6):e389-e400.
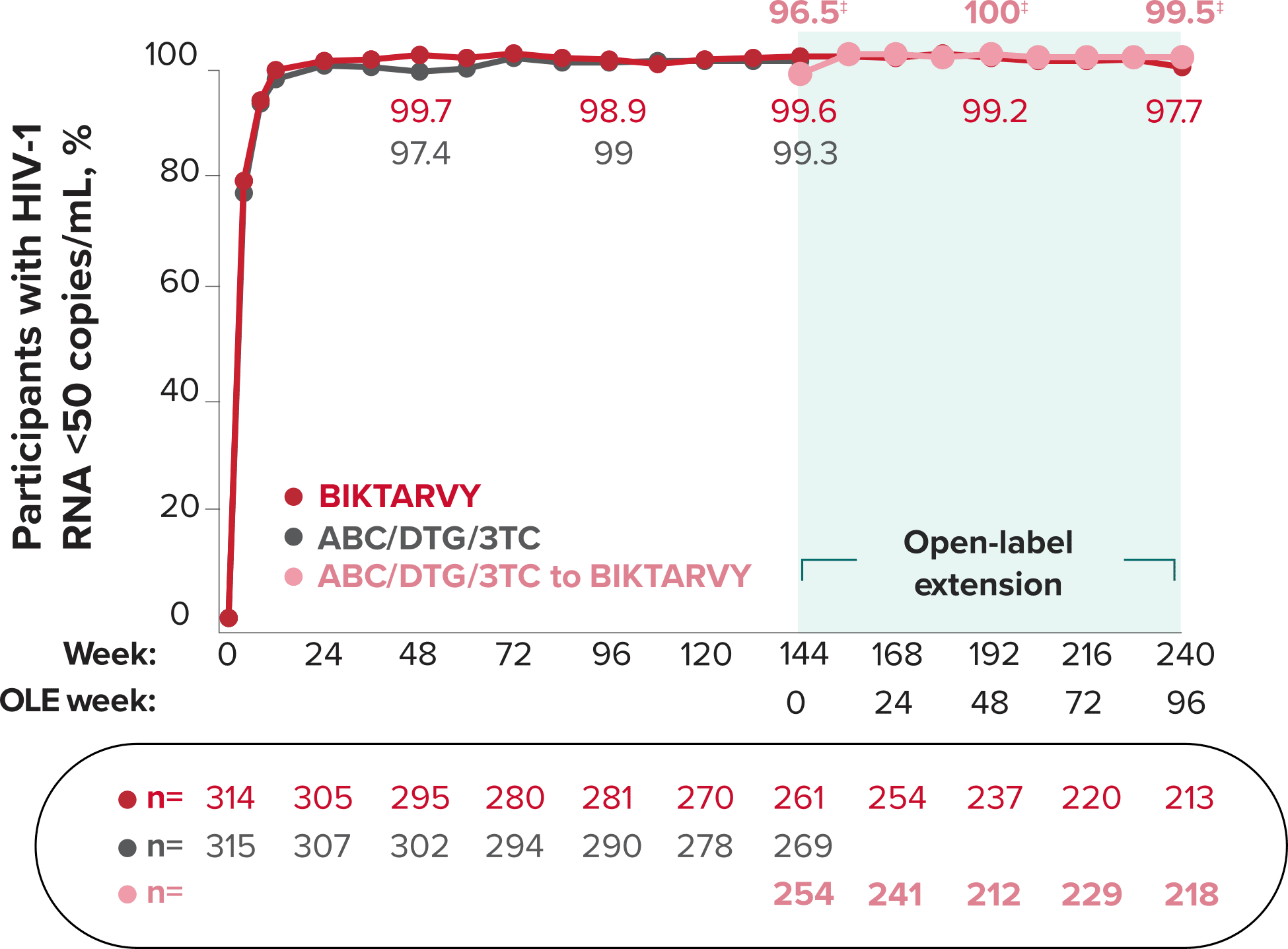
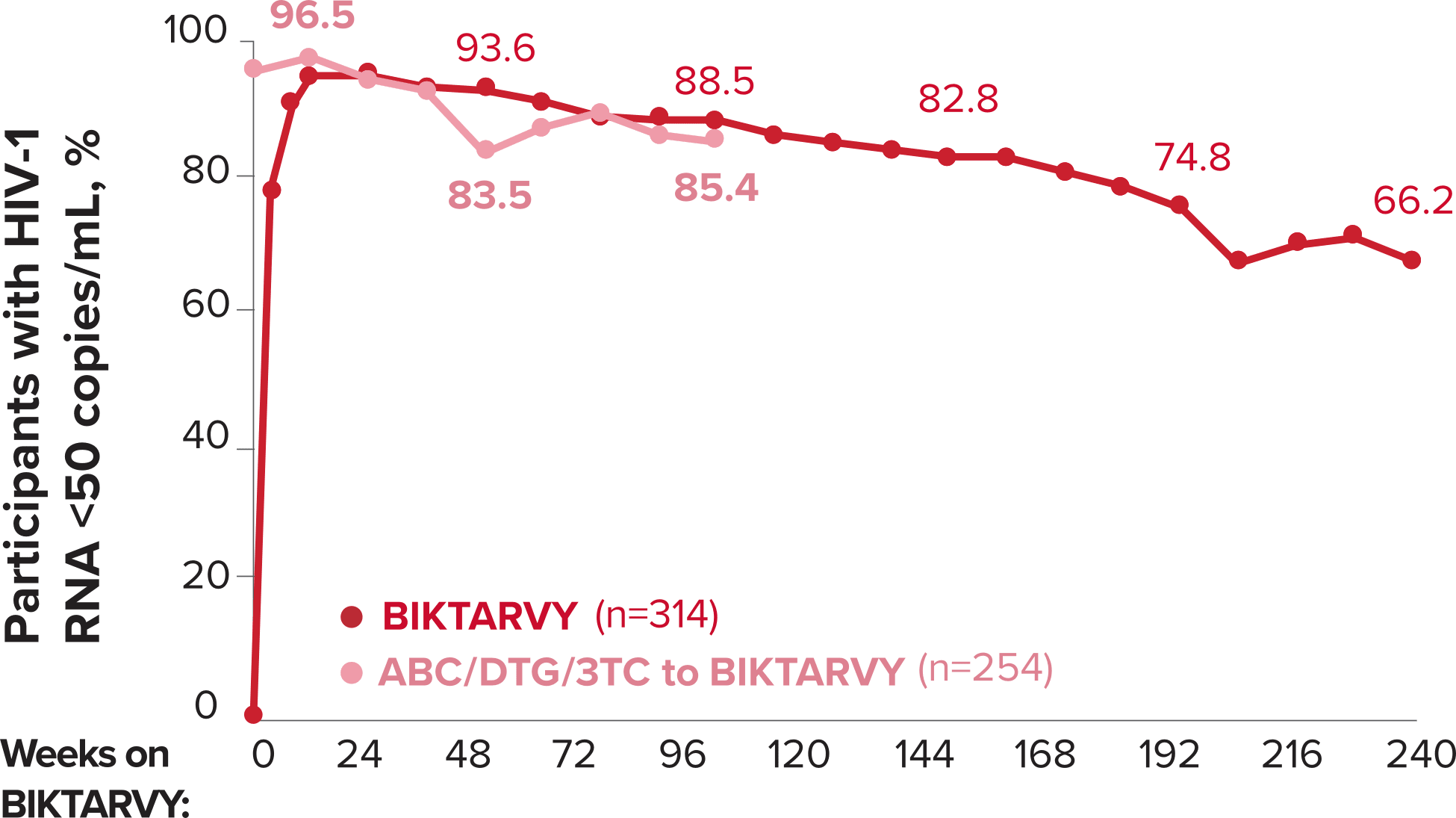
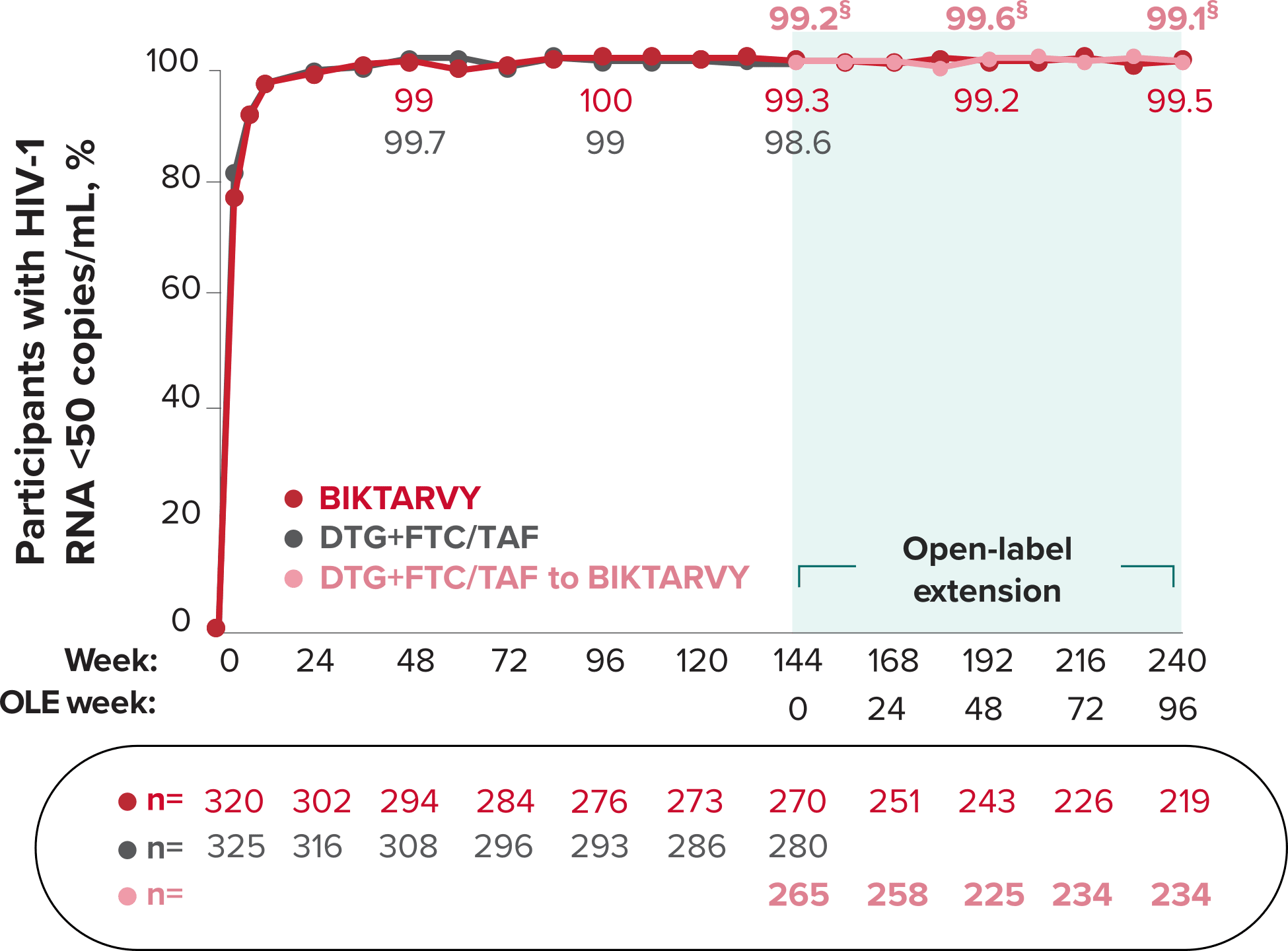
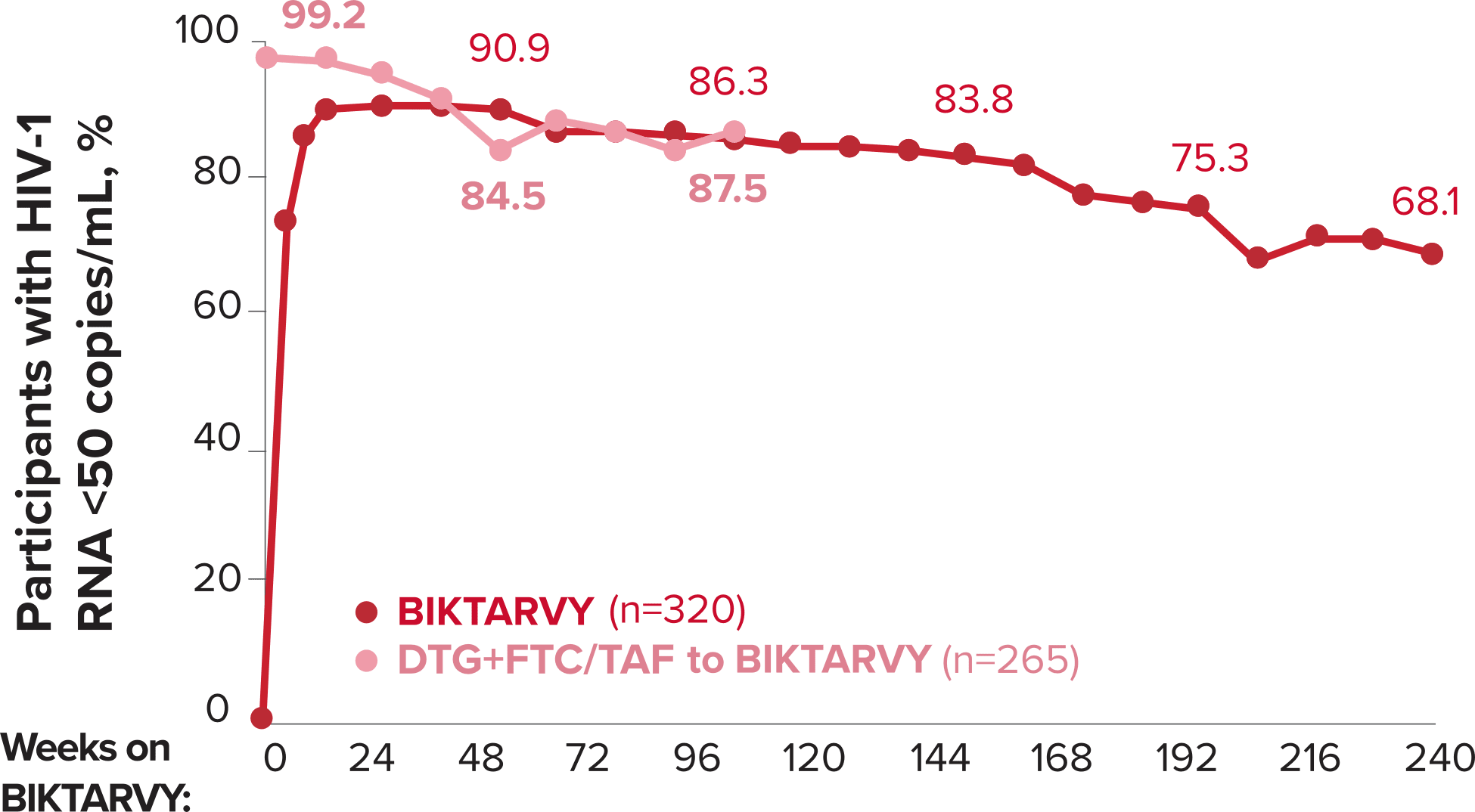
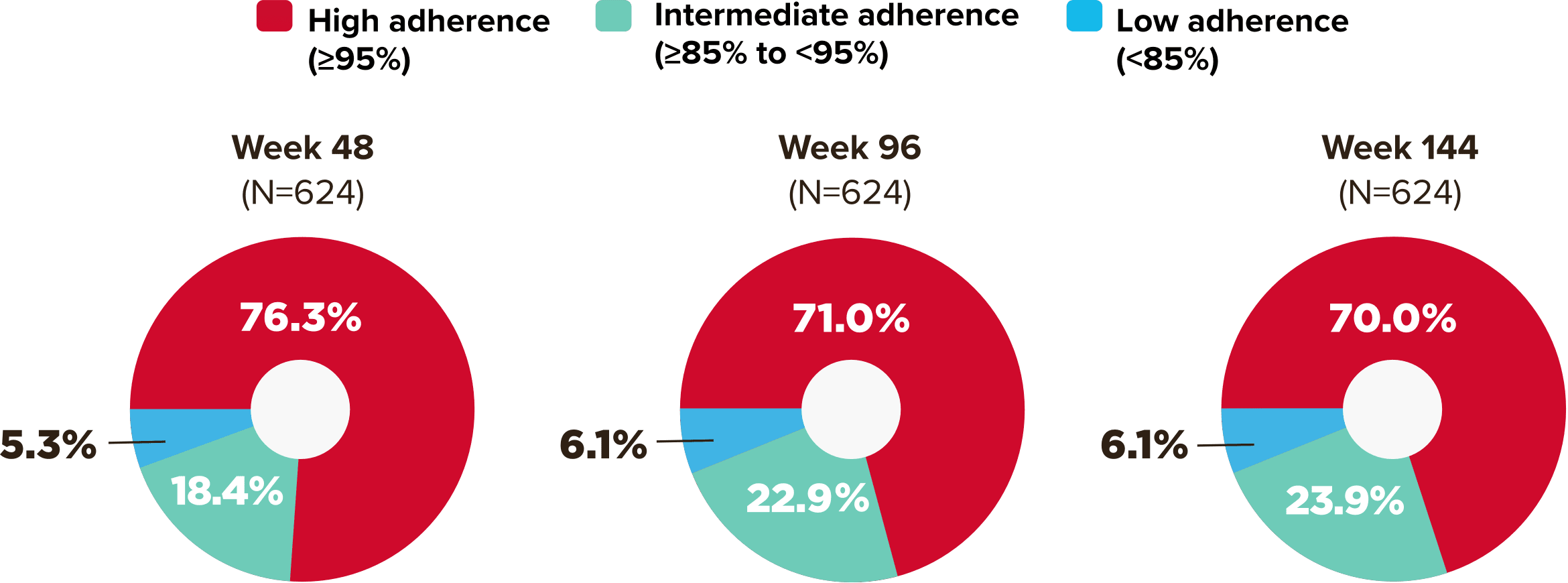
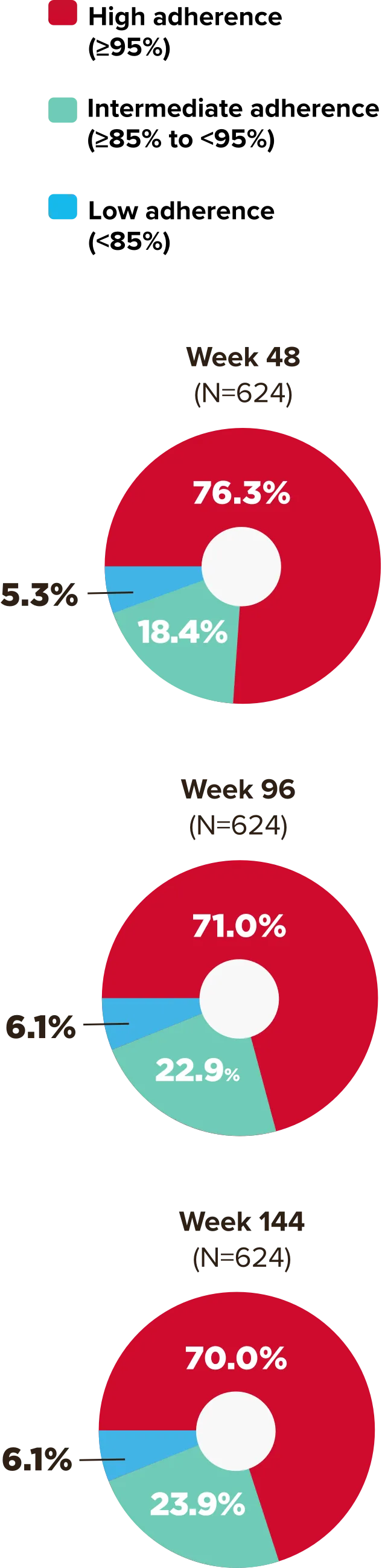
Studies 1489 and 1490: Resistance
Zero cases of resistance to BIKTARVY through 5 years in treatment-naïve adults1,2,7
Among the treatment-naïve adults who participated in Studies 1489 and 1490, 634 participants received BIKTARVY through Week 144 of the double-blind phase, and 1025 participants received BIKTARVY through Week 96 of the extension phase. Of the 1025 treatment-naïve adults who participated in the OLE, 506 participants continued on BIKTARVY, 254 participants switched from ABC/DTG/3TC, and 265 participants switched from DTG+FTC/TAF at Week 144.1,2,7
In the final resistance analysis population, no amino acid substitutions associated with BIKTARVY resistance emerged in the 11 participants who experienced treatment failure and had evaluable genotypic resistance data.2


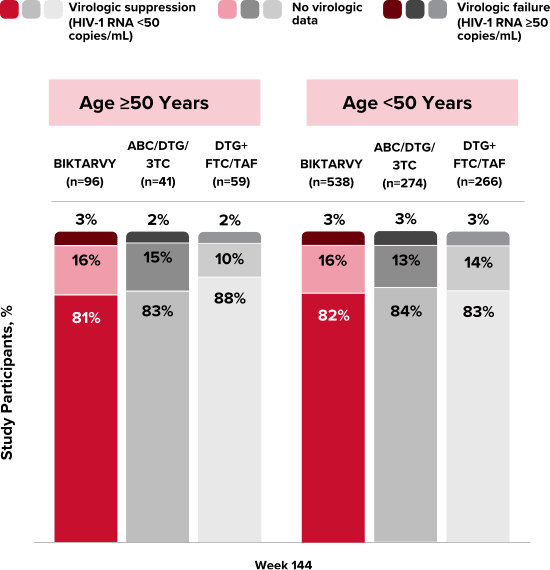
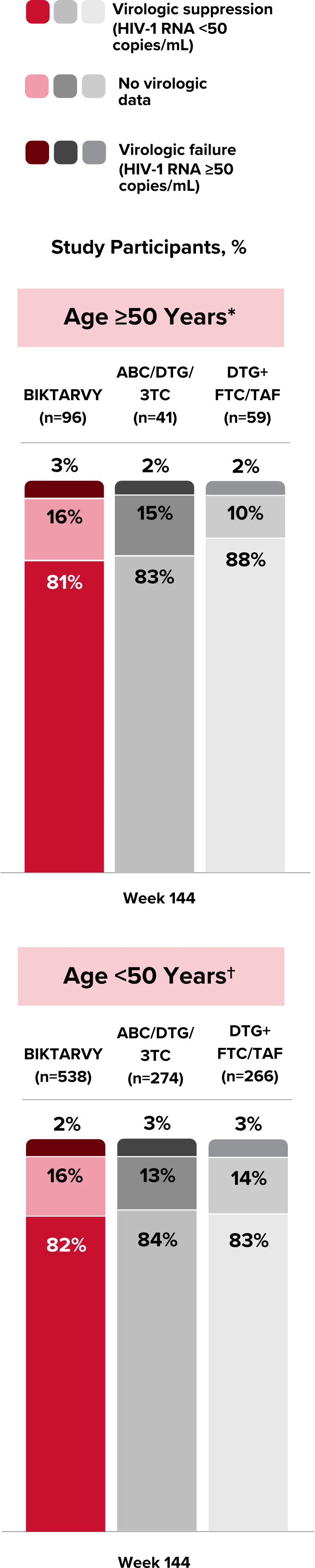
People With HIV Aged 50 and Older
Explore BIKTARVY clinical trial data.